- Title
-
Consensus guidelines for the use and interpretation of angiogenesis assays
- Authors
- Nowak-Sliwinska, P., Alitalo, K., Allen, E., Anisimov, A., Aplin, A.C., Auerbach, R., Augustin, H.G., Bates, D.O., van Beijnum, J.R., Bender, R.H.F., Bergers, G., Bikfalvi, A., Bischoff, J., Böck, B.C., Brooks, P.C., Bussolino, F., Cakir, B., Carmeliet, P., Castranova, D., Cimpean, A.M., Cleaver, O., Coukos, G., Davis, G.E., De Palma, M., Dimberg, A., Dings, R.P.M., Djonov, V., Dudley, A.C., Dufton, N.P., Fendt, S.M., Ferrara, N., Fruttiger, M., Fukumura, D., Ghesquière, B., Gong, Y., Griffin, R.J., Harris, A.L., Hughes, C.C.W., Hultgren, N.W., Iruela-Arispe, M.L., Irving, M., Jain, R.K., Kalluri, R., Kalucka, J., Kerbel, R.S., Kitajewski, J., Klaassen, I., Kleinmann, H.K., Koolwijk, P., Kuczynski, E., Kwak, B.R., Marien, K., Melero-Martin, J.M., Munn, L.L., Nicosia, R.F., Noel, A., Nurro, J., Olsson, A.K., Petrova, T.V., Pietras, K., Pili, R., Pollard, J.W., Post, M.J., Quax, P.H.A., Rabinovich, G.A., Raica, M., Randi, A.M., Ribatti, D., Ruegg, C., Schlingemann, R.O., Schulte-Merker, S., Smith, L.E.H., Song, J.W., Stacker, S.A., Stalin, J., Stratman, A.N., Van de Velde, M., van Hinsbergh, V.W.M., Vermeulen, P.B., Waltenberger, J., Weinstein, B.M., Xin, H., Yetkin-Arik, B., Yla-Herttuala, S., Yoder, M.C., Griffioen, A.W.
- Source
- Full text @ Angiogenesis
|
Endothelial cell proliferation assays. |
|
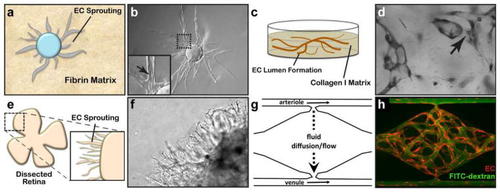
Three-dimensional assays of vascular morphogenesis. |
|
Aortic ring assay of angiogenesis. |
|
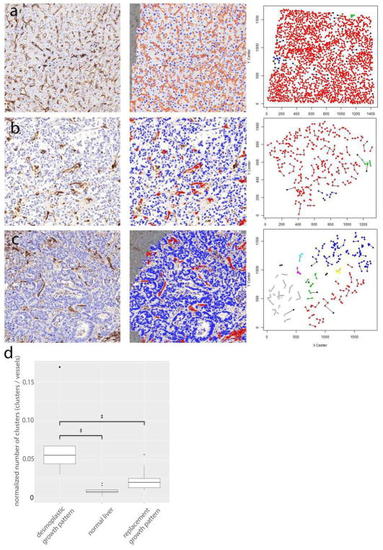
Microvessel density and histopathological growth patterns. |
|
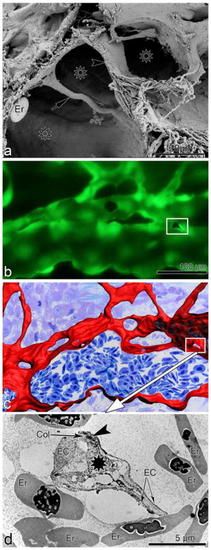
Intussusceptive angiogenesis—the methodological challenge. |
|
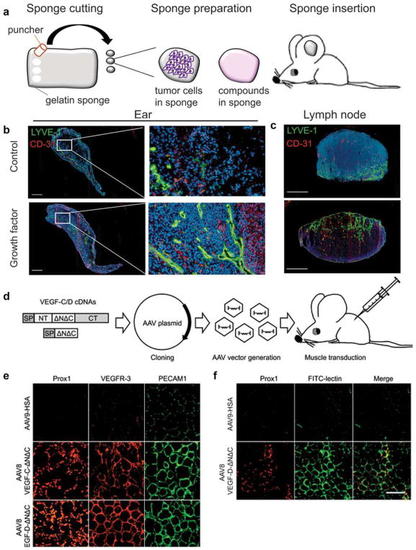
Stimulation of lymphatic and blood vessel growth in vivo. |
|
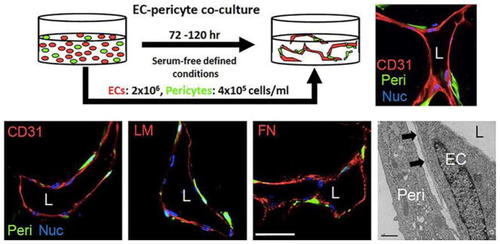
Serum-free defined model of human endothelial cell-pericyte tube co-assembly in 3D collagen matrices. |
|
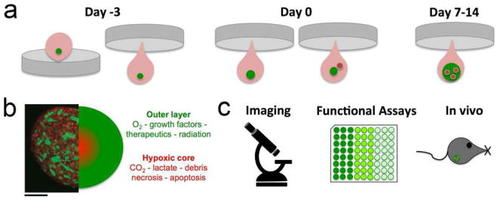
EC co-culture spheroid assay. |
|
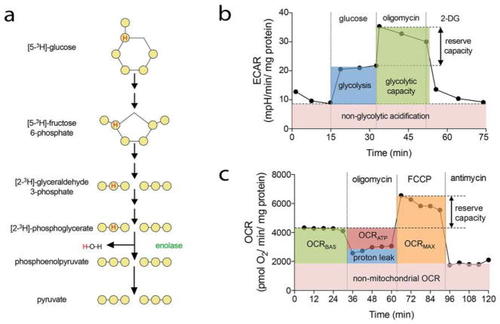
Methods to measure EC metabolism using radioactive tracers and Seahorse XF analyzer. |
|
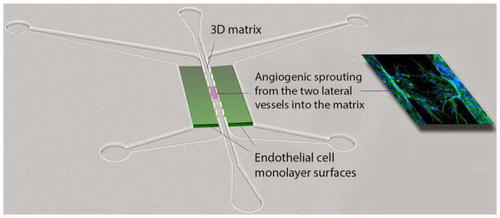
PDMS microfluidic device for analyzing angiogenesis. Fluid flow can be controlled connecting syringe pumps to the ports, or by imposing hydrostatic gradients. Flow can be directed through the endothelial lumens (green), or across the endothelial junctions, through the central matrix gel. Sprouting occurs through the apertures that flank the central 3D matrix and is easily visualized and quantified (Adapted from [ |
|
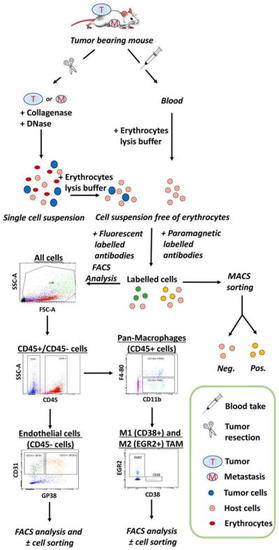
Representative flow-chart of flow cytometry and cell sorting experiments. Overview of samples collection (tumors, organs bearing metastasis, and blood) from mice and the procedure to obtain a single-cell suspension after tissue dissociation, erythrocytes lysis, and cell labeling with fluorescent or paramagnetic-coupled antibodies. Analysis of blood-circulating cells only requires a red blood cell lysis prior to incubation with antibodies of interest. Intracellular antigens can be detected by adding a cell permeabilization step. Fluorescent labeled cells are analyzed by flow cytometry and sorted by FACS while cells labeled with paramagnetic-coupled antibodies can be sorted by MACS. MACS can be used to pre-enrich cells for subsequent FACS sorting. Cell populations of interest are identified in by dot plots analysis of collected data |
|
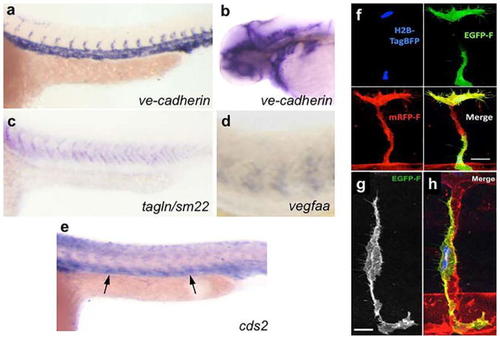
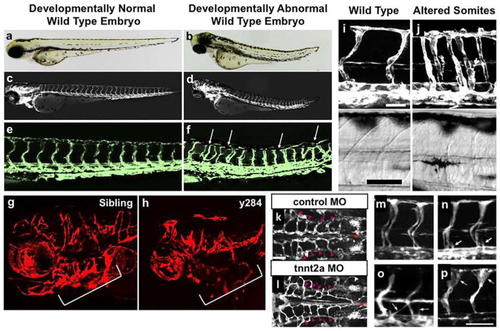
Assessing EC autonomous gene function in zebrafish. |
|
Assessing embryonic morphology and effects on vascular patterning. |
|
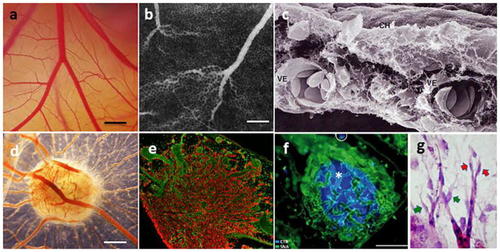
Chorioallantoic membrane of the chicken embryo (CAM). |
|
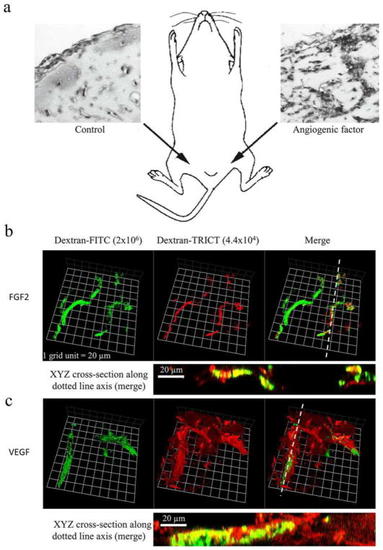
In vivo BME/Matrigel plug assay in mice. Injection of BME/Matrigel in the groin/abdomen areas of a mouse. The left image is a plug without growth factors; the right image represents a plug with an angiogenic growth factor. |
|
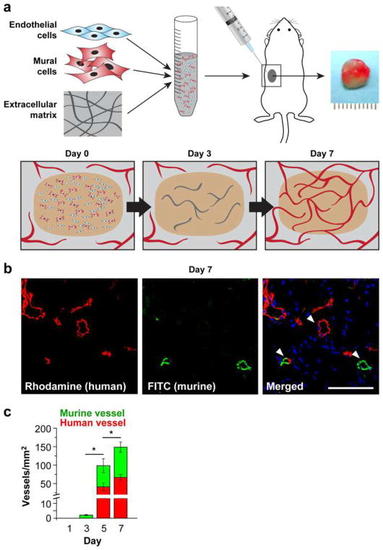
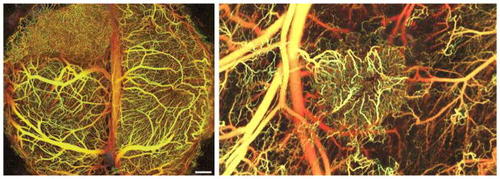
In vivo vascular network formation assay. |
|
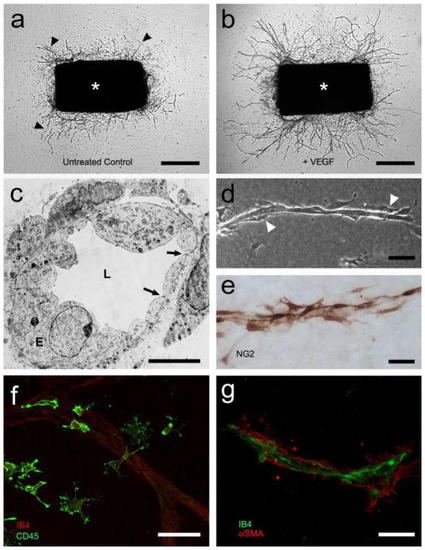
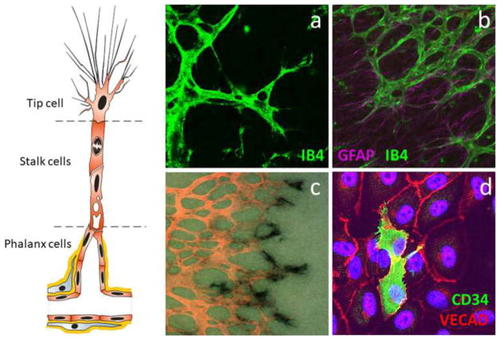
Identification of tip cells. The tip cell is the leading cell of an angiogenic sprout with long filopodia extensions, followed by stalk cells that proliferate and phalanx cells that form a matured new capillary. |
|
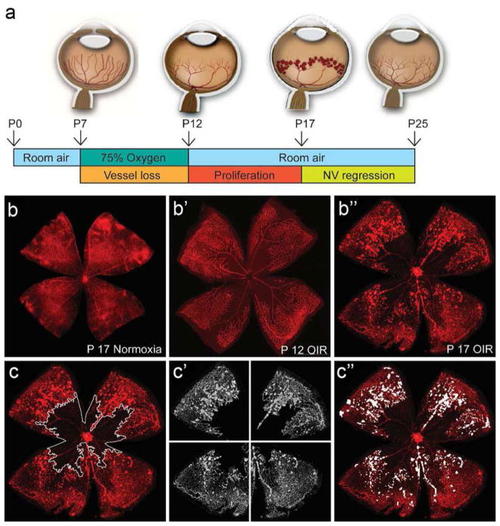
Mouse model of oxygen-induced retinopathy (OIR). |
|
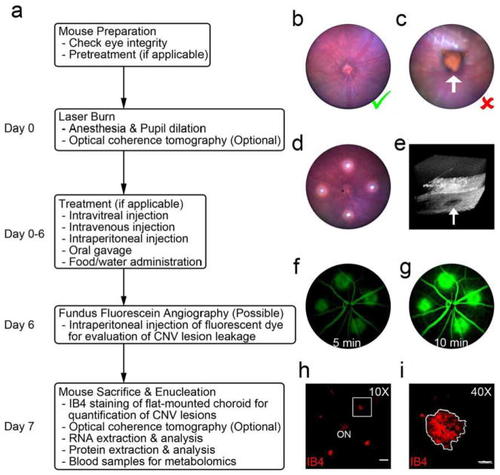
Experimental flowchart of the image-guided laser-induced CNV model and data collection. |
|
Tumor angiogenesis imaging. Vasculature in the brain (left) and the dorsal skin (right) visualized using IR frequencies to image deeper into tissue; blood flow creates the contrast, so it is noninvasive (from [ |
|
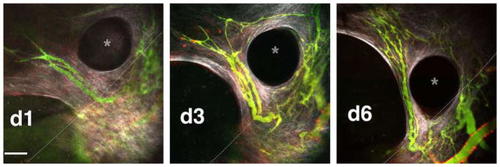
MMTV tumor vasculature in the cranial window pillar TIC. |
|
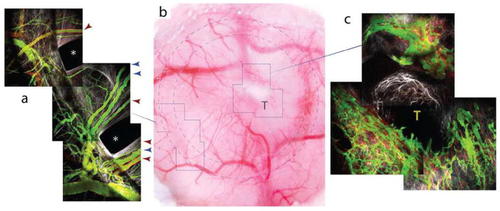
Imaging of vasculature in the cranial window preparation. Vascular sprouts entering a cranial window tissue isolation chamber. Time sequence of new vasculature (green) migrating toward the top left into a cranial window TIC, past the edge of the PDMS disk (dashed line) (imaged using MPLSM and SHG). Alignment of collagen fibers (white) is evident, and alpha-SMA+ cells can be seen on the PDMS surface (red). The vasculature (green, FITC-dextran) extends by forming perfused loops and sprouts. As the matrix remodels, the vessels also remodel as they advance. |
|
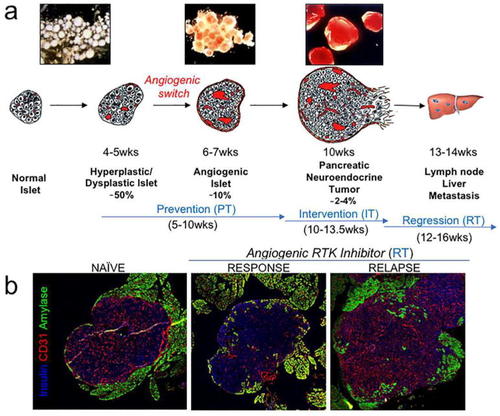
RIP1-Tag2 mouse model. |
|
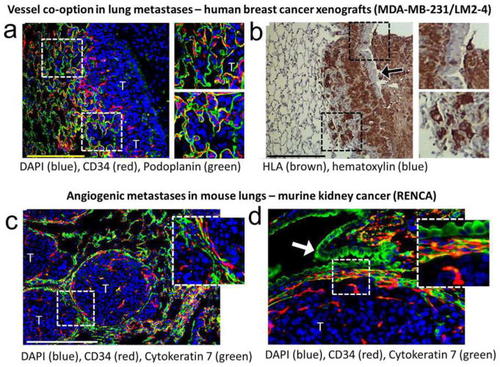
Visualization of vasculature in transplanted mouse models. Immunofluorescence and immunohistochemical staining of forma-lin-fixed mouse lung samples to enable differentiation between vessel co-option and angiogenesis in tumors. |
|
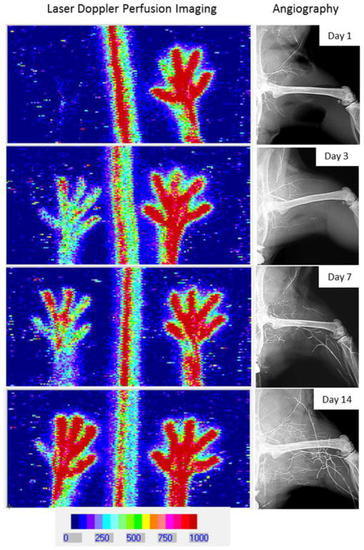
Laser Doppler perfusion imaging and angiography of blood flow. Analysis of blood flow recovery in time by laser Doppler perfusion imaging (left panels) and angiography (right panels) |
|
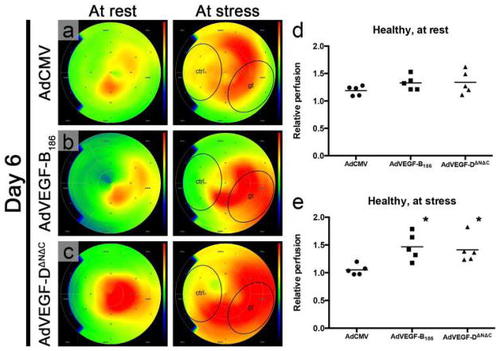
Perfusion imaging is crucial in detecting functional changes in the vasculature. In normoxic pig myocardium 6 days after intramyocardial AdVEGF-B186 and AdVEGF-DΔNΔC gene transfer, myocardial perfusion is increased at stress conditions in the treated region (gt) as measured with PET. Color scale is absolute; darkest blue is 0 ml/min/g, green is 1.5 ml/min/g, and deepest red is 3.0 ml/min/g or over ( |
|
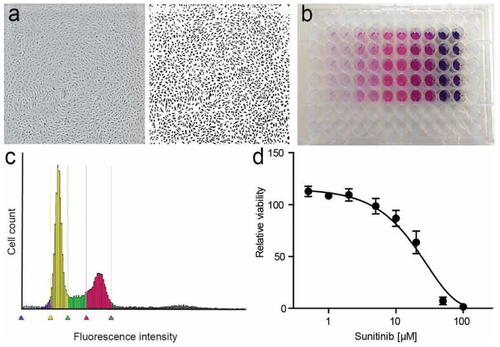
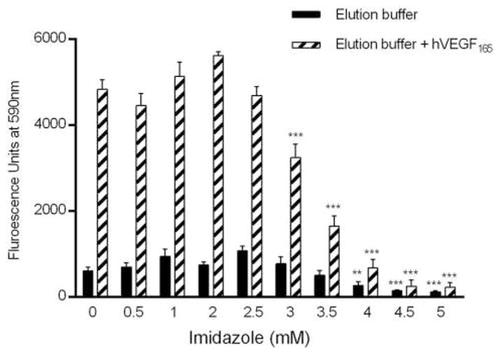
Effects of imidazole on endothelial cell proliferation. BCECs (bovine choroidal microvascular endothelial cells) were cultured in the presence of low-glucose DMEM in the presence of 10% bovine calf serum as previously described [ |