- Title
-
Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer
- Authors
- Yee, N.S., Zhou, W., and Liang, I.C.
- Source
- Full text @ Dis. Model. Mech.
|
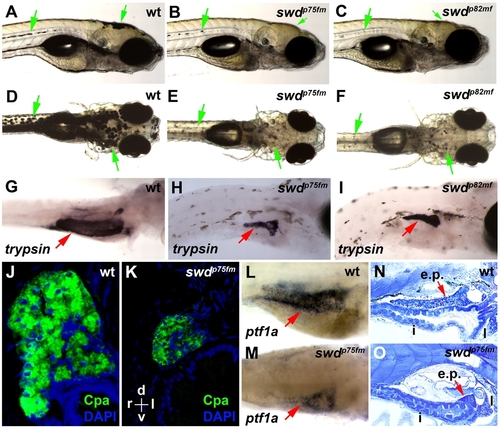
The zebrafish swdp75fm and swdp82mf mutations cause exocrine pancreatic hypoplasia and reduced skin pigmentation. (A–F) Bright-field images of the swd mutants and wild-type (wt) larvae. Green arrows point to pigmented skin. (G–I) Exocrine pancreas (red arrows) revealed by whole-mount in situ hybridization using anti-trypsin riboprobes. (J,K) Histological transverse sections of exocrine pancreas following immunohistochemistry using anti-Cpa antibodies. Staining with DAPI was used to visualize the nuclei. The histological sections are oriented as indicated: d, dorsal; v, ventral; r, right; l, left. (L,M) Exocrine pancreas (red arrows) revealed by whole-mount in situ hybridization using anti-ptf1a riboprobes. (N,O) Sagittal histological sections of larvae. e.p., exocrine pancreas; i, intestine; l, liver. (G,L) wt larvae were grown in E3 medium supplemented with PTU, which inhibits skin pigmentation, in order to facilitate visualization of the exocrine pancreas expressing trypsin or ptf1a. Note that the larvae shown in A–O were analyzed on 5 dpf. (A–C,G–I,L–O) The larvae are positioned in the same orientation and viewed from the right lateral side. (D–F) The views are in the dorsoventral direction. |
|
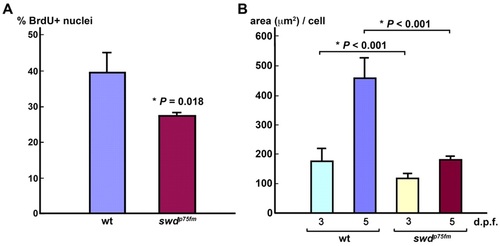
The swdp75fm mutation impairs exocrine pancreatic epithelial cell division and cell growth. (A) Cell-cycle analysis of the exocrine pancreatic epithelia in swdp75fm mutants and WT siblings at 72 hpf. % BrdU+ nuclei represent the proportion of cells in the S phase of the cell cycle. (B) Morphometric analysis of cell growth (area, in µm2, per cell) in the exocrine pancreatic epithelia of swdp75fm mutants and WT siblings on 3 and 5 dpf. Each value represents the mean + s.e.m. Statistical analysis was performed using Student’s t-test, with *P<0.05 considered statistically significant. PHENOTYPE:
|
|
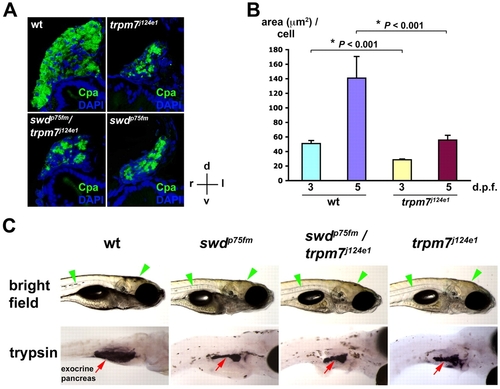
The swd locus is allelic with the trpm7 gene. (A) Exocrine pancreas of WT, the trpm7j124e1 mutant, the swdp75fm/trpm7j124e1 mutant and the swdp75fm mutant on 5 dpf by immunohistochemistry using anti-Cpa antibodies followed by cross-sectional histological analysis. Staining with DAPI was used to visualize the nuclei. The histological sections are oriented as indicated: d, dorsal; v, ventral; r, right; l, left. (B) Morphometric analysis of exocrine pancreatic epithelial growth (area, in μm2, per cell) in the trpm7j124e1 mutants and WT siblings on 3 and 5 dpf. Each value represents the mean + s.e.m. Statistical analysis was performed using Student’s t-test, with *P<0.05 considered statistically significant. (C) Bright-field images in right lateral view and whole-mount in situ hybridization using anti-trypsin riboprobes. The swdp75fm/+ and trpm7j124e1/+ heterozygotes were crossed with each other, and the progeny larvae were analyzed on 5 dpf. The WT, but not mutant, larvae were grown in medium supplemented with PTU, which inhibits skin pigmentation, in order to facilitate visualization of the exocrine-pancreas-expressing trypsin. Note the regions of skin pigmentation (green arrowheads) and trypsin-expressing exocrine pancreas (red arrows) in the mutants as compared to WT. EXPRESSION / LABELING:
PHENOTYPE:
|
|
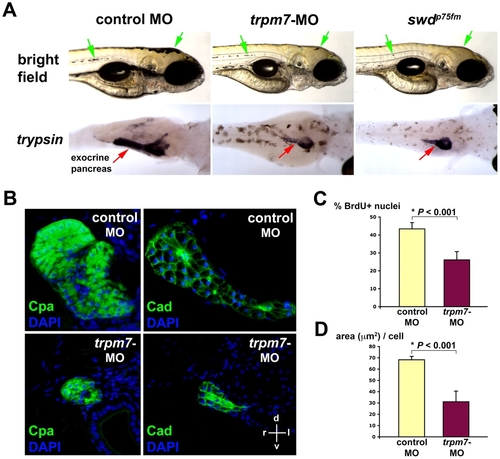
Antisense inhibition of zebrafish trpm7 expression phenocopies the swd mutation. (A) Bright-field images (right lateral view) and whole-mount in situ hybridization using anti-trypsin riboprobes (viewed in the dorsoventral direction) at 96 hpf. Note the region of pigmented skin (green arrows) and trypsin-expressing exocrine pancreas (red arrows) in the trpm7-ATG-MO-injected larvae as compared with the control. For in situ hybridization, the control-MO-injected embryos were grown in medium supplemented with PTU, which inhibits skin pigmentation, in order to facilitate visualization of the trypsin-expressing exocrine pancreas. This experiment was performed three times, with 25 embryos being injected for each group in each experiment. About 80–90% of larvae in each experiment exhibit the phenotype shown. The residual skin pigmentation is probably due to incomplete knockdown of trpm7 by the MO. (B) Acinar size and morphology revealed by immunohistochemistry using anti-Cpa or anti-cadherin (Cad) antibodies, followed by histological analysis at 96 hpf. Staining with DAPI was used to visualize the nuclei. The histological sections are oriented as indicated: d, dorsal; v, ventral; r, right; l, left. (C) Proportion of exocrine pancreatic epithelia in the S phase of the cell cycle (% BrdU+ nuclei) at 72 hpf. (D) Pancreatic epithelial cell growth (area in μm2 per cell) by morphometric analysis at 96 hpf. Each value represents the mean + s.d. *P<0.05 indicates statistically significant difference. EXPRESSION / LABELING:
PHENOTYPE:
|
|
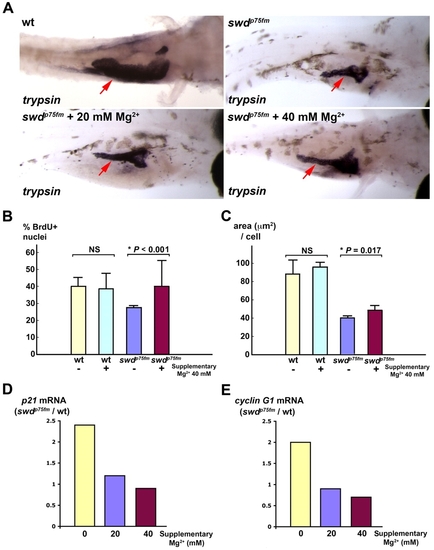
Supplementary Mg2+ partially rescues the growth defect of exocrine pancreas in swd mutants by improving cell-cycle progression and cell growth, with repression of p21cdkn1a and cyclin G1. Exocrine pancreas of the swdp75fm mutants and WT siblings incubated in medium with or without supplementary 20 mM or 40 mM MgCl2 was analyzed. The swdp75fm mutants were identified on the basis of their hypopigmented skin, which was minimally affected by the supplementary MgCl2. (A) Exocrine pancreas (red arrows) in the larvae of 5 dpf embryos by in situ hybridization using anti-trypsin riboprobes. The WT embryos were grown in medium supplemented with PTU, which inhibits skin pigmentation and facilitates visualization of the trypsin-expressing exocrine pancreas. Each larva shown is representative of 40 larvae in each experimental group, and this experiment was performed three times with similar results. (B) Proliferation assay to determine the proportion of BrdU+ nuclei (cells in S phase) in the exocrine pancreas at 72 hpf. (C) Morphometric analysis of exocrine pancreatic cell growth (area, in μm2, per cell) at 5 dpf. Each value represents the mean of five larvae + s.d. *P<0.05 is considered statistically significant. NS, not statistically significant. (D,E) Relative mRNA levels of p21cdkn1a and cyclin G1 by quantitative real-time PCR at 5 dpf. Each value represents the ratio of p21cdkn1a or cyclin G1 mRNA in the swd mutants to WT in the same experimental group. This experiment was repeated with reproducible results. |
|
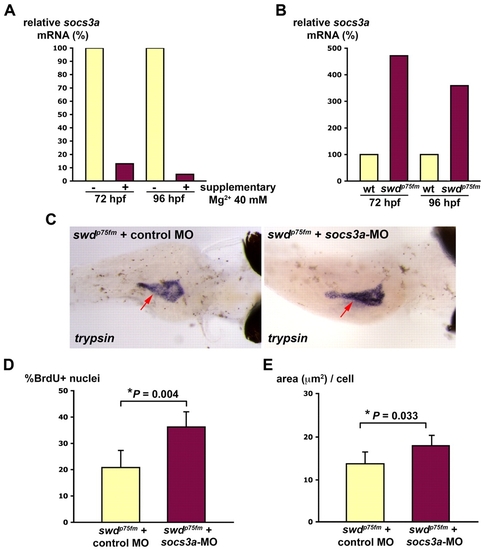
The Mg2+-sensitive Socs3a pathway is involved in the proliferative defect of the swd mutants. Supplementary Mg2+ improves the exocrine pancreas phenotype of the swd mutants that have repression of socs3a. (A) swdp75fm mutants were incubated in E3 medium with or without supplementary 40 mM MgCl2 for 72 or 96 hpf and analyzed for socs3a mRNA by real-time PCR. (B) swdp75fm mutants and WT were incubated in E3 medium (containing 1.65 mM Mg2+) for 72 or 96 hpf, and the socs3a mRNA levels were determined by real-time PCR. (C) Embryos collected from swdp75fm/+ intercross were microinjected with socs3a-ATG-MO or control MO, incubated until 4 dpf and the exocrine pancreas (red arrows) was analyzed by whole-mount in situ hybridization using anti-trypsin riboprobes. The image is representative of 20 larvae in each group from three independent experiments. The swdp75fm mutants injected with either non-targeting control MO or socs3a-5-mispair MO are indistinguishable in the exocrine pancreas by in situ hybridization using anti-trypsin riboprobes. (D) socs3a-ATG-MO- or control-MO-injected larvae were incubated until 72 hpf, injected with BrdU and then analyzed for the proportion of cells in S phase (% BrdU+ nuclei). (E) socs3a-ATG-MO- or control-MO-injected larvae were incubated until 96 hpf and analyzed for cell growth (area, in μm2, per cell). *P<0.05 indicates statistically significant difference. (C-E) The swdp75fm mutants were identified on the basis of their hypopigmented skin, which was not grossly affected by socs3a-ATG-MO. |
|
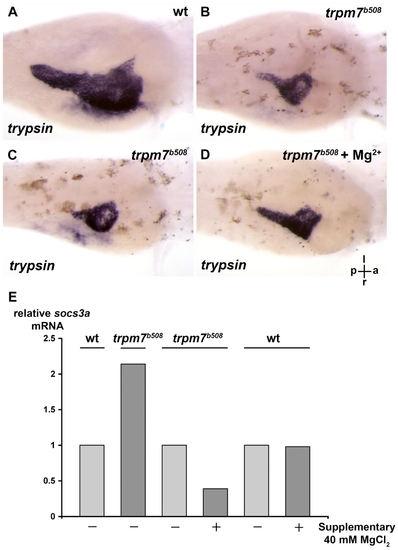
The exocrine pancreas of the trpm7b508 mutant is relatively small, and the defect can be improved by supplementary Mg2+. (A,B) The trpm7b508 mutants and their wt siblings were incubated till 96 h.p.f. and exocrine pancreas analyzed by in situ hybridization using anti-trypsin riboprobes. The wt embryos were grown in medium supplemented with PTU that inhibits skin pigmentation and facilitates visualization of the exocrine pancreas expressing trypsin. ( |
|
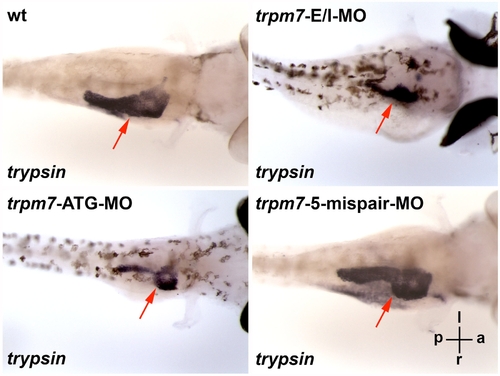
The exocrine pancreas of the swd and trpm7 mutants can be phenocopied by trpm7-E/IMO or trpm7-ATG-MO. The wt embryos injected with non-targeting control MO, trpm7-E/I-MO, trpm7-ATG-MO, or trpm7-5-mispair-MO, were incubated till 96 h.p.f. and analyzed by in situ hybridization using anti-trypsin riboprobes. The wt larvae injected with trpm7-5-mispair-MO appear indistinguishably from those injected with non-targeting control MO, such that for in situ hybridization, they were grown in E3 medium containing PTU that inhibits skin pigmentation, thus facilitating visualization of the exocrine pancreas expressing trypsin. Each larva shown is representative of 10 mutant larvae in each experimental group, and this experiment was repeated with similar results. The orientation of the larvae is indicated: a, anterior; p, posterior; l, left; r, right. The larvae are viewed in the dorsal-ventral direction. The red arrows indicate the trypsin-expressing exocrine pancreas. |
|
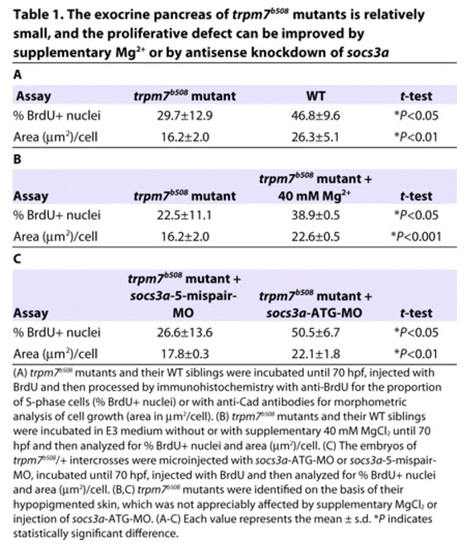
The exocrine pancreas of trpm7b508 mutants is relatively small, and the proliferative defect can be improved by supplementary Mg2+ or by antisense knockdown of socs3a. PHENOTYPE:
|