- Title
-
Zebrafish dou yan mutation causes patterning defects and extensive cell death in the retina
- Authors
- Catalano, A.E., Raymond, P.A., Goldman, D., and Wei, X.
- Source
- Full text @ Dev. Dyn.
|
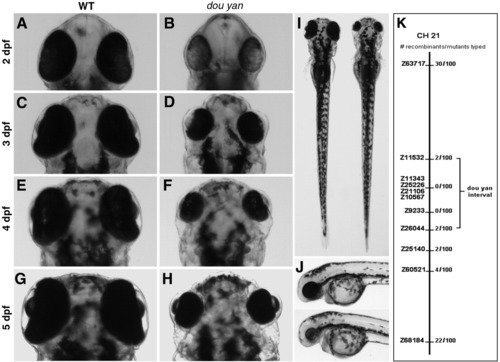
The zebrafish dou yanmi234 mutation causes reduction in eye size. A-H: The small eye phenotype in the mutants is evident at 2 days postfertilization (dpf). The mutant eyes remain smaller than the wild-type eyes at subsequent developmental stages. I: The dou yanmi234 mutation does not lead to reduction of body size in mutants. In a dorsal view at 4 dpf, the body of this mutant fish (right) is slightly longer than that of a wild-type sibling (left). J: A lateral view of a wild-type (top) and mutant fish (bottom) at 3 dpf shows no paracardiac edema in dou yan mutants. K: A bulk segregant mapping analysis revealed that the dou yan gene localizes to a genomic region that is flanked by markers Z11532 and Z26044 on chromosome 21. |
|
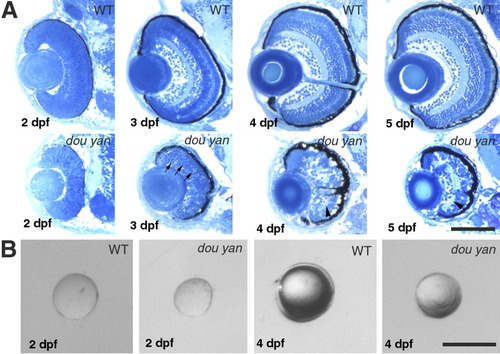
The dou yanmi234 mutation disrupts cytoarchitecture of the retina and reduces the retinal size more severely than it does to the lens. A: Histological analyses sections of wild-type and mutant retinas at 2, 3, 4, and 5 days postfertilization (dpf) reveal that retinal lamination is severely disrupted in the mutant eyes. Arrows indicate the darkly stained pyknotic nuclei in the mutant retina. Arrowheads indicate the disrupted plexiform layers. B: The isolated lenses of mutant embryos are 6% and 30% smaller than their counterparts of wild-type embryos at 48 hours postfertilization (hpf) and 4 dpf, respectively. Scale bars = 100 μm. |
|
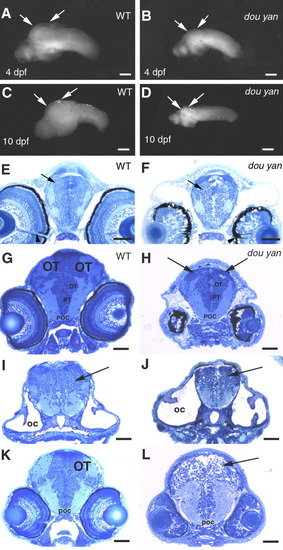
The dou yanmi234 mutation preferentially affects the development of the optic tectum more than it does the other regions of the brain. A,B: Lateral views of the isolated wild-type (A) and mutant (B) brains at 4 days postfertilization (dpf) confirm that most regions of the mutant brain are only slightly smaller than those of the wild-type brain. However, the optic tectum is drastically reduced in the mutant embryos (arrows). C,D: The arrows indicate the optic tectum regions of brains isolated from wild-type (C) and surviving mutants (D) at 10 dpf. E,F: Cross-sections of the heads of wild-type (E) and mutant (F) embryos at the optic nerve region indicate similar forebrain size at 4 dpf, with the dorsal-ventral diameter of the mutant forebrains (arrows) being approximately 94% of that of the wild-type brains. The overall patterning of the gray (cell bodies and nuclei) and white (neuronal processes) matter is similar between the mutant and the wild-type embryos. Arrowheads indicate the optic nerves, which serve as a reference point for comparable section planes. G,H: The cross-sections of 4 dpf wild-type (G) and mutant (H) heads through the midbrain region reveal that the optic tectum (OT) is almost missing in the comparable region in the mutant embryos (arrows, H). However, the posterior commissure (POC), posterior tuberculum (PT), and the dorsal thalamus (DT) are comparable between mutant and wild-type embryos. I,J: The cellular patterning of the hindbrain mutant (J) is similar to that of the wild-type brain (I) at 4 dpf. Arrows indicate the hindbrains. The otic capsules (OC) were selected as a reference point for comparable section planes. K,L: At 3 dpf, cross-sections through the midbrain revealed the optic tectum in wild-type embryos (K). However, no optic tectum-like structure is evident in the mutant embryos (L) at the corresponding region (arrow). The posterior commissure (POC) is selected as a reference point for matching comparison. Scale bars = 100 μm in A,B,C-G, 50 μm in E,F,G-L. PHENOTYPE:
|
|
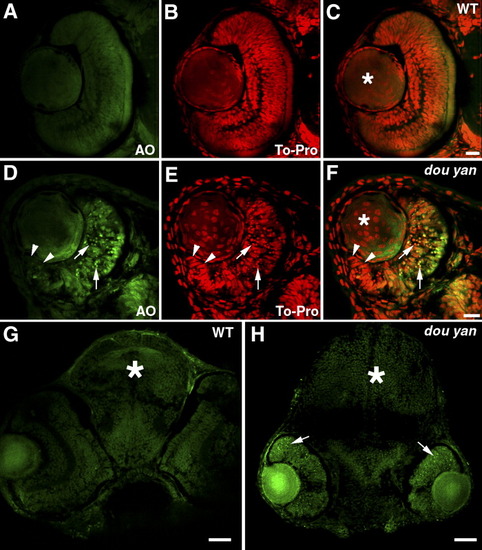
The dou yanmi234 mutation causes extensive cell death in the retina but not in the brain or lens. A-F: Staining with acridine orange (AO, green), which preferentially stains the nuclei of dead or dying cells, indicates extensive cell death in the central regions of the mutant retinas (D-F, arrows) compared with the wild-type retinas (A-C). The cell nuclei are stained with TO-PRO-3 (red). Cell nuclei with an elongated shape are found at the marginal zone of the mutant retina (D-F, arrowheads), similar to the germinal zone of proliferating cells found at the retinal margin in wild-type retinas at this stage (A-C). In the lenses (asterisks), no increase in cell death was observed in dou yan. However, nonspecific acridine orange staining is sometimes observed in non-nuclear regions in the lens. G,H: Cell nuclei that are heavily stained with acridine orange are mainly found in the mutant retina (arrows) but not in the brain (asterisks). Scale bars = 20 μm in A-F, 50 μm in G,H. |
|
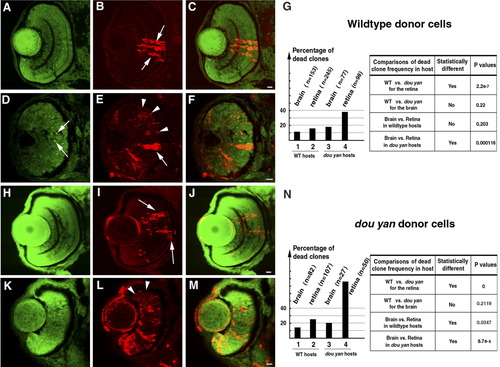
The dou yanmi234 mutation causes non-cell-autonomous retinal cell death. A-C: Clones of dextran-labeled wild-type donor cells (red) in the wild-type retina form vertical columns of cells with a differentiated morphology that span all three retinal nuclear layers (arrows). The cell nuclei were stained with YO-PRO-1 (green); C is the merged image of A and B. D-F: Extensive punctate staining of donor cells (red, arrowheads in E) was observed in the mutant retinas, indicating that many wild-type donor cells were already dead at the time of fixation. Some donor cells appeared intact in the mutant retina, but they had an elongated morphology typical of undifferentiated retinal progenitors (E, arrow). Pyknotic nuclei heavily stained with YO-PRO-1 are indicated with arrows in D. G: A histogram illustrates the percentages of dead wild-type donor clones in four host conditions: in wild-type brains, in wild-type retinas, in mutant brains, and in mutant retinas. The total number of clones counted in each condition is indicated in parentheses. Comparison of the percentages of dead donor cell clones with the χ2 test indicates that the increase in dead wild-type donor clones in the mutant retina, but not the brain, is statistically significant. H-J: Healthy-appearing dou yan mutant donor cells (red, arrows) develop properly in the wild-type retina and can localize to all three retinal cellular layers. The cell nuclei are counterstained with YO-PRO-1 (green). K-M: Large amounts of punctate staining foci derived from mutant donor cells (arrowheads) were observed in mutant host retinas, indicating that many of the donor cells were already dead at the time of fixation. N: A histogram illustrates the percentages of dead clones of mutant donor cells in four host conditions: in wild-type brains, in wild-type retinas, in mutant brains, and in mutant retinas. The total numbers of clones counted in each condition are indicated in parentheses. Comparison of the percentages of dead donor cell clones with the χ2 test indicates that statistically more mutant donor cells survived in the wild-type retinas than in the mutant retinas at the time of examination. Scale bars = 10 μm. |
|
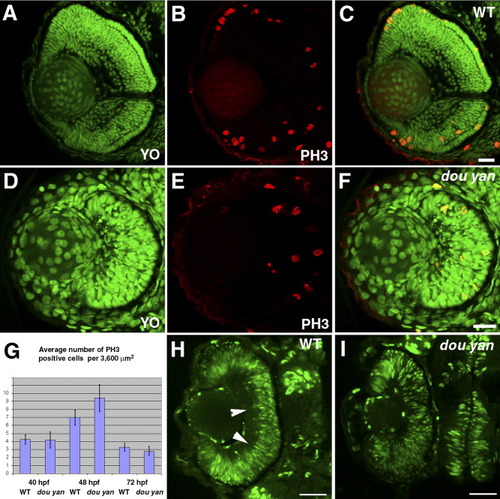
The dou yanmi234 mutation does not disrupt cell proliferation in the retina.A-F: M-phase nuclei, visualized by anti-phospho-Histone 3 antibody (PH3, red), are observed in both wild-type (A-C) and mutant retinas (D-F) at 3 days postfertilization (dpf). All nuclei are stained with YO-PRO-1 iodide (YO, green). C is the merged image of A and B. F is the merged image of D and E. G: The average number of PH3-positive cells per 3,600 μm2 in mutant retinas is similar to wild-type retinas at 40 hours postfertilization (hpf), 48 hpf, and 72 hpf. The error bars denote the standard error of the mean. H: Bromodeoxyuridine (BrdU) labeling of the wild-type retina between 49 hpf and 51.5 hpf reveals proliferating cells in most regions of the retina. Arrowheads indicate BrdU-negative basal regions in the wild-type retina. I: BrdU labeling of dou yan mutants between 49 hpf and 51.5 hpf confirms that many retinal cells are actively synthesizing DNA. Scale bars = 20 μm. |
|
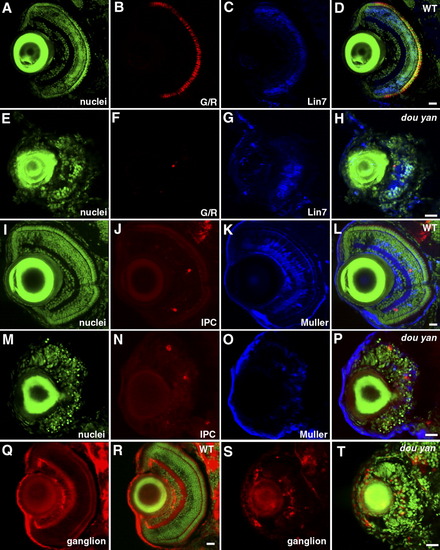
Distinct types of retinal cells are specified in dou yanmi234 mutants, but they fail to differentiate properly. A-H: At 5 days postfertilization (dpf), green/red double cones (G/R, B and F) and Lin7-positive bipolar cells (Lin7, C and G) are observed in both mutant (E-H) and wild-type (A-D) retinas. YO-PRO staining was used to visualize the entire population of cell nuclei (nuclei, A,E). D and H are merged images. I-P: Interplexiform cells (IPC, J,N) and Muller cells (Muller, K and O) are specified in both wild-type (I-L) and mutant retinas (M-P) at 5 dpf. Cell nuclei were visualized by YO-PRO staining (I,M). L and P are merged images. Q-T: Zn8 (red) -expressing ganglion cells are specified in both wild-type (Q,R) and mutant (S,T) retinas. Cell nuclei were visualized with YO-PRO staining (green). Scale bars = 20 μm. EXPRESSION / LABELING:
|
|
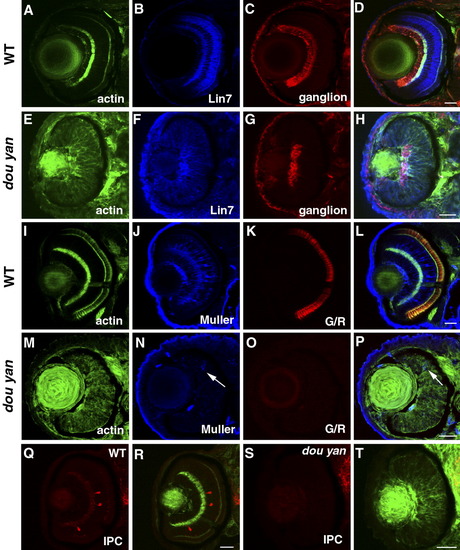
Retinal cell specification is delayed by the dou yan mutation. A-D: At 78 hours postfertilization (hpf) in wild-type retinas, Lin7 expression (blue) is concentrated in the bipolar cell body region and the plexiform layers. The ganglion cells stained with zn8 antibody (red) localize to the basal region of the retinas. The actin distribution as revealed by phalloidin staining highlights the plexiform layers. E-H: In dou yan mutant retina at 78 hpf, Lin7 (blue) is expressed in the entire retina with increased accumulation in the basal retinal region. This expression pattern is similar to Lin7 expression in wild-type retinas at 48 hpf (Wei et al.,[2006a]). A patch of retinal cells that express zn8 antigen is noticeable at the basal region of the retina in the mutants. I-L: Muller cells (blue) and green/red double cones (G/R, red) are properly differentiated in the wild-type retinas at 78 hpf. M-P: In most of the mutant retinas at 78 hpf, only a few cells display positive expression of Muller cell marker carbonic anhydrase (arrows, blue) and the double cone marker Zpr1 was not detectable. Q-T: Interplexiform layer cells (IPC, red) are present in wild-type retinas (Q,R) but not in the mutant retina (S,T) at 78 hpf. Scale bars = 20 μm. EXPRESSION / LABELING:
|
|
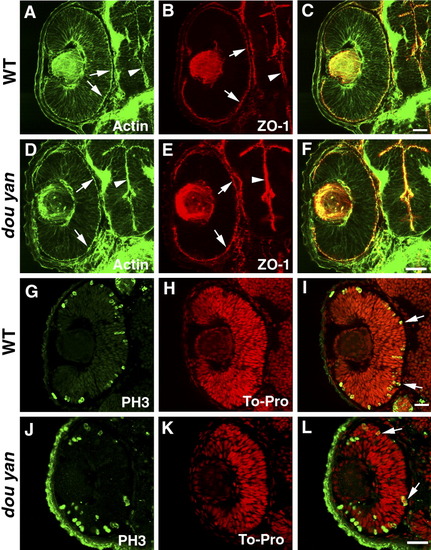
The dou yanmi234 mutation does not affect apical epithelial polarity. A-C: Apical localization of adherens junctions in the retina (arrows) and brain (arrowheads) of the 48 hours postfertilization (hpf) wild-type embryos is visualized by the staining patterns of ZO-1 (red) and adherens junction-associated actin bundles (A, green, arrows). D-F: ZO-1 (red) and adherens junction-associated actin bundles (green) localize properly to the apical surface of the retina (arrows) and brain (arrowheads) in dou yanmi234 embryos at 48 hpf. G-L: In 48 hpf retinas, the M-phase nuclei as visualized with anti-phosphorylated-Histone H3 antibody (PH3, green) localize to the apical regions of the retinas of mutant (J-L) and wild-type (G-I) embryos. Cell nuclei were labeled with TO-PRO (red). Scale bars = 20 μm. EXPRESSION / LABELING:
|

Unillustrated author statements |