- Title
-
Zebrafish Neural Tube Morphogenesis Requires Scribble-Dependent Oriented Cell Divisions
- Authors
- Zigman, M., Trinh, L.A., Fraser, S.E., and Moens, C.B.
- Source
- Full text @ Curr. Biol.
|
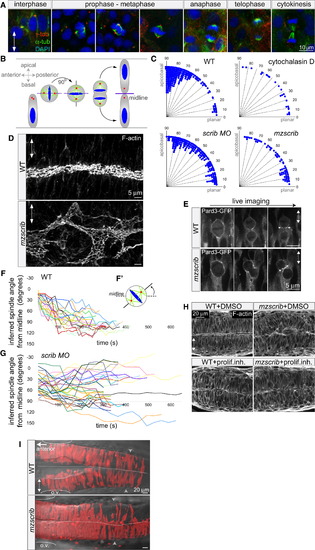
Scrib Regulates Mitotic Spindle Orientation and Neural Tube Morphogenesis (A and B) Immunostainings of neural keel progenitors (A) showing that the orientation of the mitotic spindle changes over the course of mitosis. γ-tubulin (centrosomes) is shown in red, α-tubulin (spindle) in green, and DAPI (DNA) in blue. This process results in the bilateral distribution of daughter cells as schematized in (B). (C) Quantification of mitosis orientation at anaphase. Chi-square analysis shows that the three distributions shown are highly significantly different from wild-type (WT) (n = 269): 3 μg/ml cytochalasin D-treated (n = 28, χ2 = 51, 1 degree of freedom [df]; p < 0.001), scrib morphant (n = 235, χ2 = 306, 8 df; p < 0.001), mzscrib mutant (n = 76, χ2 = 201, 2 df; p < 0.001). Although all three distributions are more homogeneous than WT, only cytochalasin D treatment results in a statistically randomized distribution (χ2 = 2.7, 3 df; p = 0.44). (D) Posterior hindbrain lumen morphology, defined by apical F-actin, with an abnormal, branched organization in the mzscrib mutant compared to WT. (E) Pard3-GFP localization to subapical foci upon cytokinesis (arrows) occurs normally in WT and misoriented mzscrib mutant neural keel progenitors. (F and G) Representation of mitotic spindle rotation in live WT (F; n = 20) and aberrantly rotating scrib morphant (G; n = 32) progenitors. Plots present the angle between inferred mitotic spindle axis and the midline over time. (F′) Scheme of spindle rotation in a mitotic progenitor. Mitotic spindle axis is shown as a solid line; midline is shown as a dashed line. (H) Inhibition of cell proliferation results in diminished neural tube morphogenesis defects in mzscrib embryos. (I) Requirement of Scrib for cross-midline cell divisions in the neural keel. Labeled cells in a 22 hours postfertilization (hpf) WT embryo have a bilateral distribution, but mzscrib mutant embryos have a predominantly unilateral distribution in the posterior hindbrain/anterior spinal cord region. Arrowheads indicate the position of the first somite; ovals mark the otic vesicle (o.v.); dotted line indicates the midline. In all panels, anterior is to the left and two-way arrows indicate the apicobasal axis of neuroepithelial progenitors. See also Figure S1. PHENOTYPE:
|
|
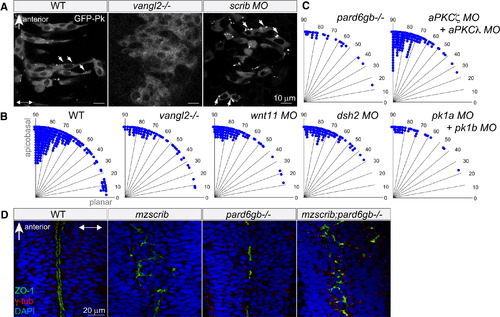
Neither Planar Cell Polarity Components nor the Par Complex Are Required for Proper Spindle Orientation in the Neural Keel (A) GFP-Prickle (GFP-Pk)-positive foci (arrows) in posterior hindbrain neural keel progenitors of WT, vangl2-/- mutant, and scrib morphant embryos. In (A) and (D), anterior is to the top and two-way arrows indicate the apicobasal axis. (B and C) Quantification of anaphase orientation without functional planar cell polarity and Par complex components. The distributions of the various mutant or morphant conditions shown are not significantly different from WT (n = 269): vangl2-/- mutant (n = 72, χ2 = 5.1, 4 df; p = 0.28), wnt11 morphant (n = 74, χ2 = 2.6, 3 df; p = 0.41), dsh2 morphant (n = 86, χ2 = 9.6, 4 df; p = 0.05), pk1a + pk1b morphant (n = 38, χ2 = 2.6, 3 df; p = 0.46), pard6gb-/- mutant (n = 28, χ2 = 2.7, 3 df; p = 0.43); the distribution of mitotic angles in aPKCζ + aPKCλ double morphants is slightly more biased toward apicobasal than WT (n = 139, χ2 = 14.4, 4 df; p = 0.006). (D) The branched, disorganized neural tube lumen of mzscrib and mzscrib;pard6gb-/- double mutants. Dorsal optical sections at 18 hpf immunostained with γ-tubulin (red), ZO-1 (green), and DAPI (blue) are shown. See also Figure S2. EXPRESSION / LABELING:
PHENOTYPE:
|
|
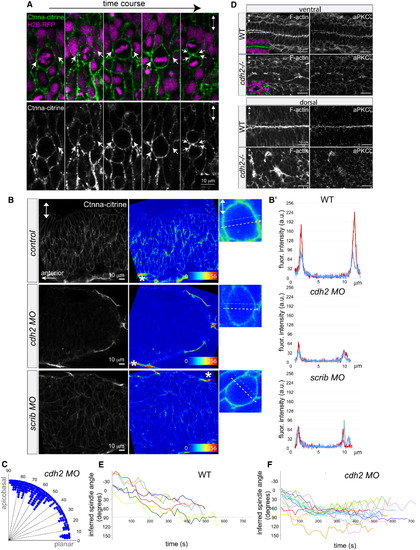
Reduction of α-Catenin Foci in the Neural Keel Correlates with Aberrant Mitotic Orientation and Neural Tube Architecture Defects (A) Equatorially positioned cortical Ctnna-citrine foci (α-catenin, green, arrows) are stationary while chromosomes (H2B-RFP, purple) rotate in a time-lapse of a dividing neural keel progenitor. Upper panel shows merge; lower panel shows Ctnna-citrine alone. (B) Scrib and Ncad/Cdh2 are required for Ctnna-citrine abundance and the localization of equatorial cortical Ctnna-citrine foci in neural keel mitotic progenitors. Posterior hindbrain is shown at 6–8 somites with Ctnna-citrine (left column), as Ctnna-citrine fluorescence intensity in pseudocolors (middle column), and in pseudocolors of single mitotic progenitors (right column). Localization of Ctnna-citrine is shown in WT (top row), cdh2 morphants (middle row), and scrib morphants (bottom row). The unchanged Ctnna-citrine signal in nonneural peridermal cells is marked by an asterisk. (B′) Fluorescence intensity plots of Ctnna-citrine levels in WT, cdh2 morphant, and scrib morphant mitotic cells (shown in B), with the y axis displaying arbitrary gray values along a line across a mitotic cell at equatorial (white dashed arrow in pictures; red line in plots) and lateral (faint blue dashed arrow in pictures; blue line in plots) positions averaged over 8 pixels in width. (C) Quantification of mitosis orientation at anaphase in cdh2 morphants. This distribution is highly significantly different from WT (n = 267, χ2 = 224, 8 df; p < 0.001). For WT controls, see Figure 1C. (D) Disorganized, branched neural tube midline in the cdh2-/- neural tube in horizontal cryosections. F-actin/phalloidin is shown in green, aPKCζ in white, and DAPI (DNA) in purple. (E and F) Representation of mitotic spindle rotation in live WT (E; n = 6) and cdh2 morphant (F; n = 16) neural keel progenitors. Spindle orientation is inferred from the orientation of the chromosomes marked with H2B-GFP. Images in all panels are in dorsal view. Two-way arrows indicate the apicobasal axis. See also Figure S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
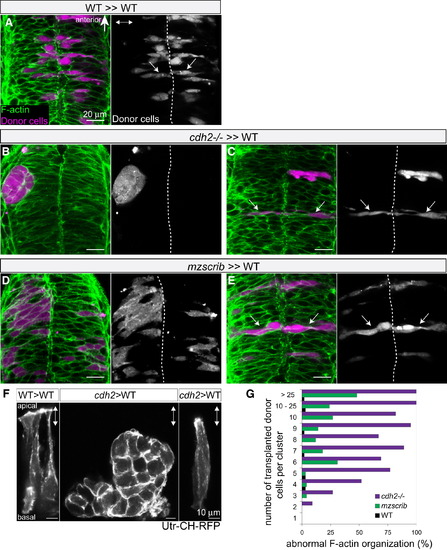
Nonautonomous Rescue of Oriented Cross-Midline Cell Division of Single cdh2-/- and mzscrib Mutant Cells (A–E) Genetic mosaics at neural tube stage (21 hpf) in which donor-derived cells (purple) were transplanted into the presumptive posterior hindbrain of WT host embryos (F-actin in green). (A) Bilateral distribution of WT cells (arrows) in a WT environment. (B) cdh2-/- cells form unilateral aggregates in a WT environment. (C) In contrast, isolated cdh2-/- progenitors show rescued bilateral distribution and normal cell shape. (D) A large group of mzscrib mutant cells in a WT environment with unilateral cell distribution. (E) Isolated mzscrib cells in a WT environment have rescued shape and bilateral distribution. (F) Utr-CH-RFP reporter in mosaic embryos showing normal apical enrichment in WT control transplants, loss of apical enrichment in a cluster of transplanted cdh2-/- cells, and rescue in a single cdh2-/- cell that is surrounded by WT host cells. Images represent maximum-intensity projections of optical sections. (G) Quantification of occurrence of abnormal F-actin organization in WT (black; n = 5 experiments, 13 embryos), mzscrib (blue; n = 3 experiments, 21 embryos), and cdh2-/- (red; n = 2 experiments, 15 embryos) mosaics. Embryos are shown in dorsal view with anterior to the top. Dotted white line indicates the neural tube midline; two-way arrows indicate the apicobasal axis of the neuroepithelium. See also Figure S4. |
|
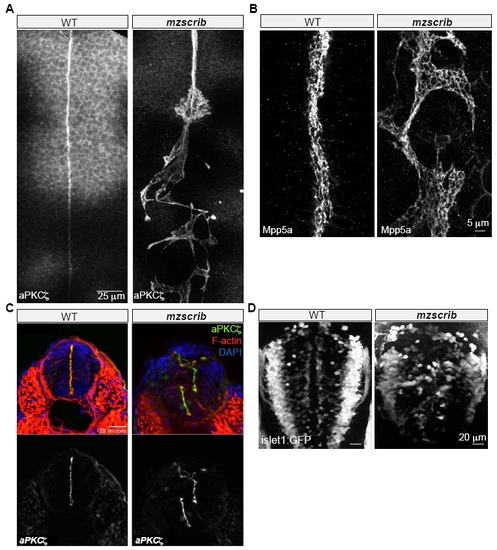
Requirement of Scrib in Zebrafish Posterior Hindbrain Organization |
|
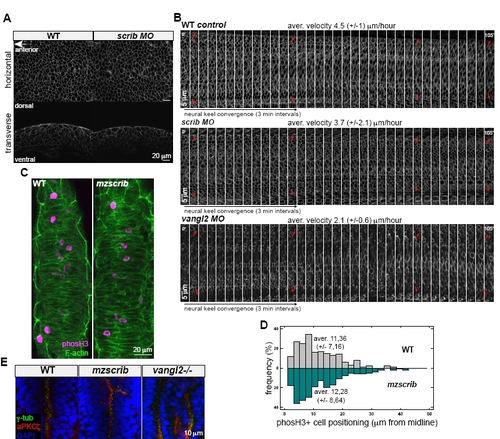
Scrib Is Not Essential for Proper Velocity of Hindbrain Neural Keel Cell Convergence, and Scrib Shows Different Effects on Zebrafish Hindbrain Organization from Vangl2 |
|
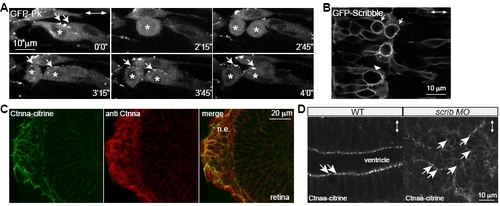
GFP-Prickle Localizes to Cytoplasm, GFP-Scrib Shows Cortical Localization in WT Mitotic Neural Keel Progenitors, and Scrib Is Not Required for Maintenance of Subapical -Catenin Localization in the Mature Neural Tube Epithelium |
|
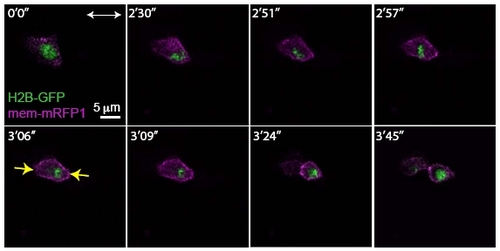
Rescue of Apicobasal Cell Division Orientation of a Single cdh2-/- Mutant Cell in a Wild- Type Environment |