- Title
-
Tbx5a lineage tracing shows cardiomyocyte plasticity during zebrafish heart regeneration
- Authors
- Sánchez-Iranzo, H., Galardi-Castilla, M., Minguillón, C., Sanz-Morejón, A., González-Rosa, J.M., Felker, A., Ernst, A., Guzmán-Martínez, G., Mosimann, C., Mercader, N.
- Source
- Full text @ Nat. Commun.
|
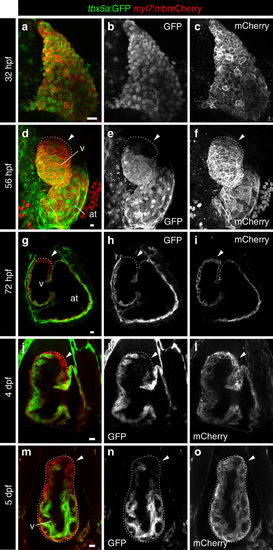
Expression profile of tbx5a-positive cardiomyocytes in embryonic zebrafish hearts. a–f Whole-mount immunofluorescence of tbx5a:GFP;myl7:mbmCherry double transgenic zebrafish hearts at 32 a–c (n = 5/5) and 56 hours postfertilisation (hpf) d–f (n = 3/3). g–l Confocal optical sections of tbx5a:GFP;myl7:mbmCherry hearts at 72 hpf g–i (n = 5/5), 4 days postfertilisation (dpf) j–l (n = 9/9), and 5 dpf (m–o; n = 6/6). GFP (green) labels tbx5a+ cells and mCherry (red) marks cells expressing the pan-myocardial marker myosin light chain 7 (myl7). Shown are ventral views, cranial is to the top. At 32 hpf all cardiomyocytes are tbx5a:GFP+ but at 56, 72 hpf, 4, and 5 dpf tbx5a:GFP− cardiomyocytes can be observed in the distal ventricle (arrowheads). The atrioventricular canal and large portions of the atrium are also GFP+. at, atrium; v, ventricle; Scale bars, 10 μm |
|
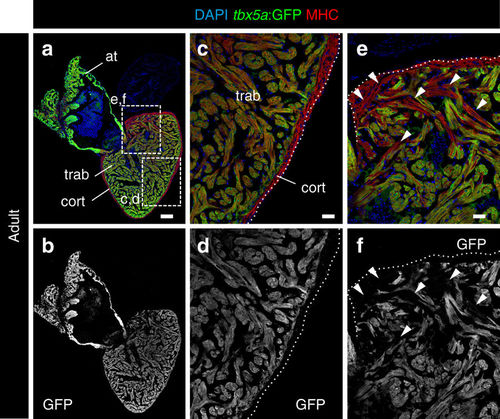
Expression profile of tbx5a-positive cardiomyocytes in adult zebrafish hearts. a, b Sagittal sections through tbx5a:GFP adult uninjured heart immunostained with GFP (green) and Myosin Heavy Chain (MHC; red). Nuclei are counterstained with DAPI (blue). c–e Zoomed views of boxed area in a. b, d, f Single channels for GFP. The trabecular myocardium is tbx5a:GFP+, whereas the cortical layer is tbx5a:GFP−. Note tbx5a:GFP− cardiomyocytes (arrowheads) in the basal part of the ventricle close to the atrioventricular canal (n = 13/13). at, atrium; cort, cortical layer; trab, trabecular layer. Scale bars, a, b 100 μm and c–f 25 μm |
|
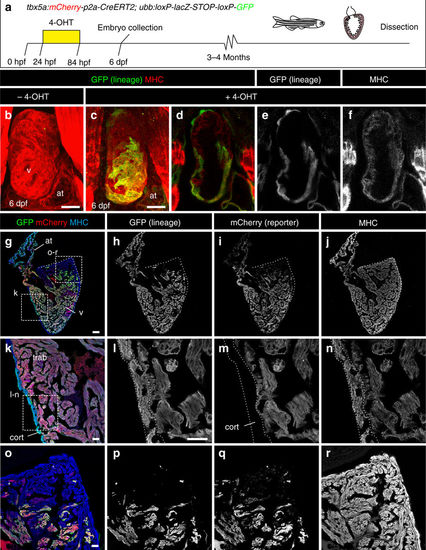
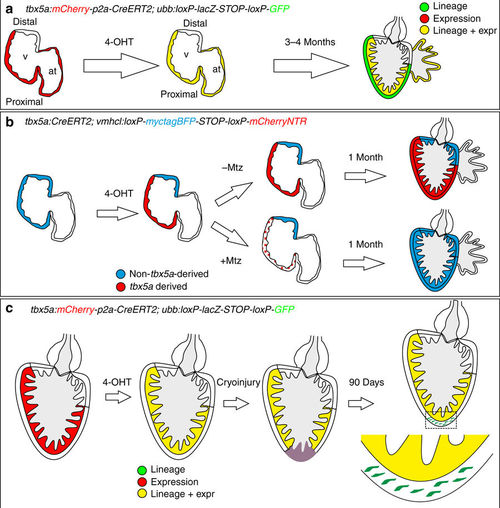
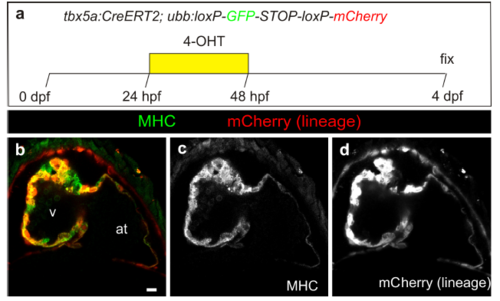
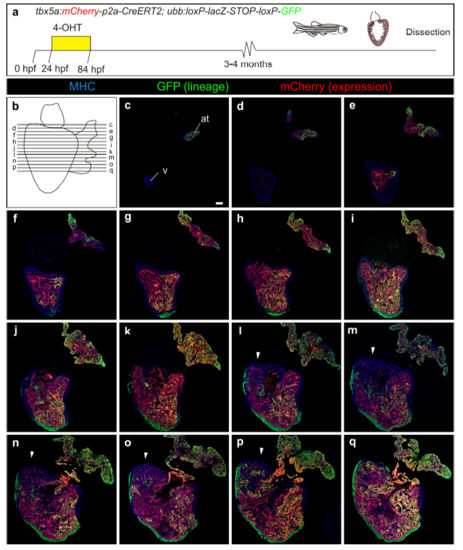
Fate mapping of tbx5a-derived cells during cardiac development. a tbx5a:mCherry-p2A-CreERT2;ubb:loxP-LacZ-STOP-loxP-GFP hearts fixed at different developmental stages. mCherry marks tbx5a-expressing cells; GFP marks tbx5a-derived cells. b–f Whole-mount ventral view of hearts at 6 days postfertilisation (dpf) stained for GFP (green) and Myosin Heavy Chain (MHC, red). b In the absence of 4- Hydroxytamoxifen (4-OHT) administration, no GFP+ cells are visible (n = 5/5). c–f 4-OHT was added from 24 to 84 hours postfertilisation (hpf). GFP expression is observed in the proximal part of the ventricle; n = 8/8. In some cases, GFP expression was also found in epicardial cells located in the distal part of the ventricle. g–r Immunofluorescence staining of adult heart sections recombined as in c. Shown are merged and single channels for GFP (green), mCherry (red), and anti-MHC staining (blue); n = 5/5. at, atrium; cort, cortical layer; trab, trabecular layer; v, ventricle. Scale bars, g 100 μm and b, c, k, l, o 25 μm |
|
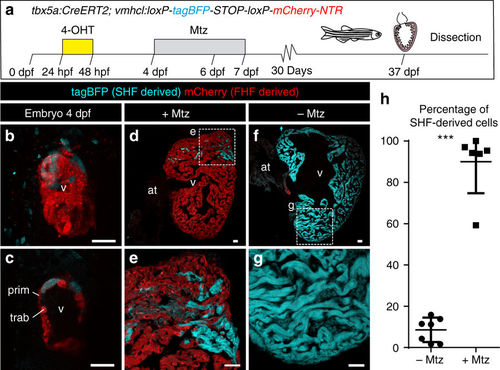
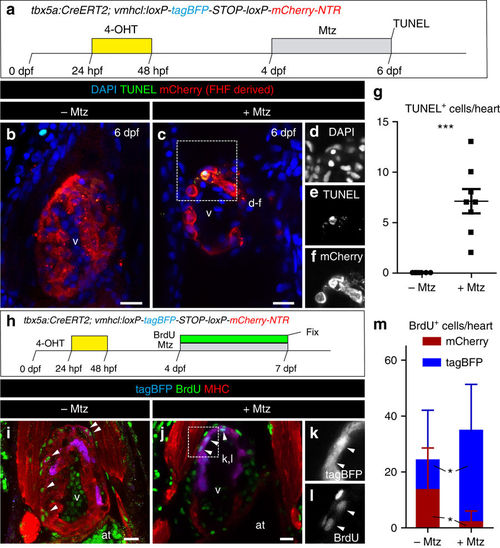
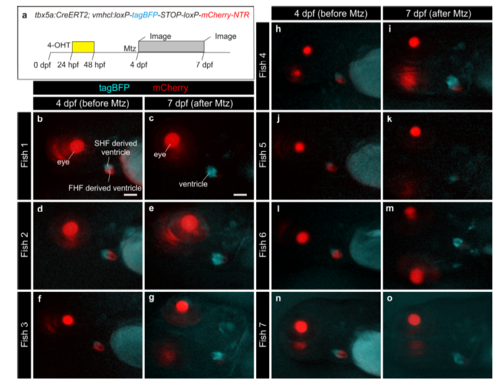
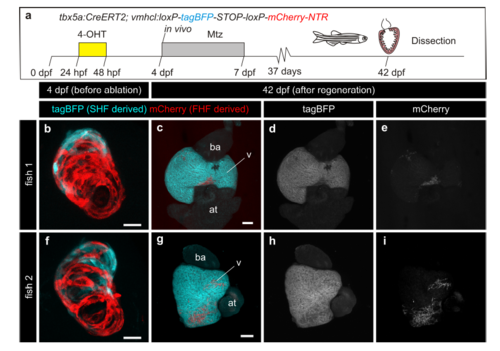
Genetic ablation of tbx5a-derived ventricular cardiomyocytes. a tbx5a+ ventricular cardiomyocytes were genetically ablated in tbx5a:CreERT2;vmhcl:loxP-tagBFP-loxP-mCherry-NTR double transgenic zebrafish. Recombination was induced by administration of 4-Hydroxytamoxifen (4-OHT). Cell ablation was induced by administration of Metronidazole (Mtz) from 4 to 7 days postfertilisation. b, c Ventral views of larval hearts at 4 dpf (maximal projection and optical section, respectively). Anterior is to the top. The proximal ventricle, including primordial layer and trabeculae, is completely mCherry+ and the distal ventricle is blue (tagBFP+); n = 7/7. d, e Sagittal section of the ventricle of an adult recombined heart. Most cells are mCherry+. Only the tbx5a− region is tagBFP. n = 7/7. f, g, Sagittal section of a Mtz-treated fish. Most of the cardiomyocytes are tagBFP+; n = 6/6. h, Quantification of the percentage of myocardium that is tagBFP+ (SHF-derived), mean±SD; *** P < 0.0001 by two-tailed unpaired t-test. at, atrium; prim, primordial layer; SHF, second heart field; trab, trabeculae; v, ventricle. Scale bars, 25 μm |
|
Apoptosis and cell proliferation upon genetic ablation of tbx5a-derived ventricular cardiomyocytes. a tbx5a+ ventricular cardiomyocytes were genetically ablated in tbx5a:CreERT2;vmhcl:loxP-tagBFP-loxP-mCherry-NTR double transgenic zebrafish. Recombination was induced by administration of 4-Hydroxytamoxifen (4-OHT). Cell ablation was induced by administration of Metronidazole (Mtz) from 4 to 7 days postfertilisation (dpf). b, c Optical sections of 6 dpf fish that had been treated with 4-OHT and Mtz as indicated in a (b) or only with 4-OHT c immunostained for mCherry (red) and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) (green). Note that some rounded mCherry+ cells are TUNEL+. d–f Single channels of selected area in c. g Quantification of the number of TUNEL+ trabecular and cortical mCherry+ cardiomyocytes per heart from animals of the +Mtz (n = 8) and –Mtz (n = 8) group, mean ± SD; ***P = 0.0004 by Mann–Whitney non-parametric t-test. h Schematic representation of the 5-Bromo-2´-deoxyuridine (BrdU) treatment to assess proliferation. i, j Fish were treated with 4-OHT and BrdU i or with 4-OHT, Mtz, and BrdU j. k, l Single channels of the boxed area in j. m Quantification of BrdU+/mCherry+ and BrdU+/tagBFP+ cells per heart. Shown are means ± SD (n = 8 for − Mtz hearts and n = 11 for + Mtz hearts, from two separate independent experiments) *P = 0.0240 for tagBFP+ cells and P = 0.0371 for mCherry+ cells by two-tailed t-test. at, atrium; hpf, hours postfertilization; v, ventricle. Scale bars, 25 μm |
|
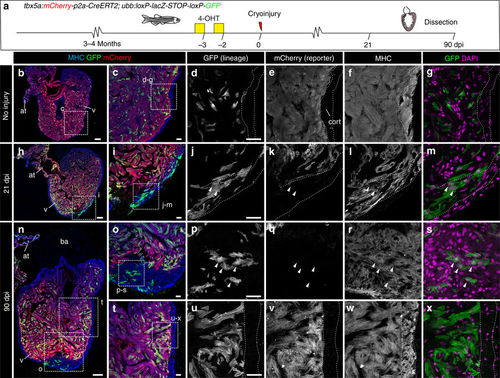
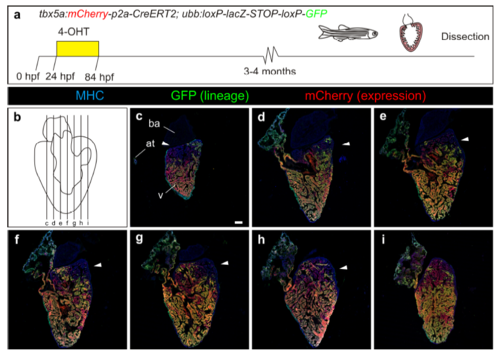
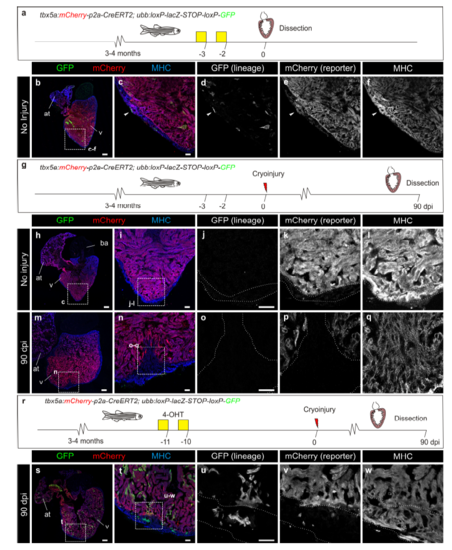
Contribution of tbx5a-derived cells during regeneration of the adult zebrafish heart. a tbx5a:Cherry-p2A-CreERT2 was crossed into ubb:loxP-lacZ-STOP-loxP-GFP. 4-Hydroxytamoxifen (4-OHT) was added 2 and 3 days before cryoinjury, to induce recombination of loxP sites. Hearts were fixed at 21 and 90 days postinjury (dpi) and sectioned for immunofluorescent detection of GFP+ tbx5a-derived cells and mCherry+ tbx5a-expressing cells. Nuclei were counterstained with DAPI. b In the uninjured heart (n = 7/7), mCherry expression was homogeneous in the trabecular myocardium and absent in the cortical layer. GFP+ cells were found in the trabecular layer. c Zoomed view of boxed area in b. d–f Single channels of boxed area shown in c. g GFP and DAPI channels only. h, n Section of hearts at 21 dpi h and 90 dpi n. Upon cryoinjury to the ventricular apex, tbx5a-derived cardiomyocytes were present also in the cortical layer, particularly at the site of injury (h–m, n = 6/7; o–s, n = 5/6; t–x, n = 6/6), whereas tbx5a+ cardiomyocytes in general were restricted to the trabecular myocardium (n = 5/6) t–x. Nuclear counterstaining revealed GFP+ cell bodies in the cortical layer (arrowheads). at, atrium; cort, cortical layer; ba, bulbus arteriosus; v, ventricle. Scale bars, b, h,n 100 µm and c, d, i, j, o, p, t, u 25 μm |
|
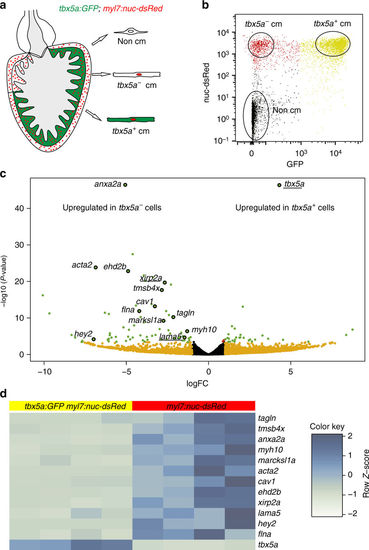
tbx5a+ and tbx5a− cardiomyocytes from adult ventricles exhibit distinct expression profiles. a, b GFP+/nuc-dsRed+ and GFP−/nuc-dsRed+ cardiomyocytes were fluorescence-activated cell (FAC) sorted from adult tbx5a:GFP;myl7:nuc-dsRed ventricles (n = 5 pooled heart per replicate; four replicates in total). c Volcano plot representing RNA-seq results comparing both populations. Black, false discovery rate (FDR) > 0.05, log fold change (LFC) < 1; orange, FDR > 0.05, LFC > 1; red, FDR < 0.05, LFC < 1; green, FDR < 0.05, LFC > 1. d Heatmap of genes differentially expressed in tbx5a+ and tbx5a– cardiomyocytes from adult hearts. Dark blue, higher expression; light blue, lower expression. cm, cardiomyocytes |
|
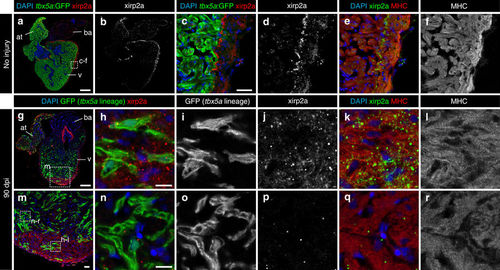
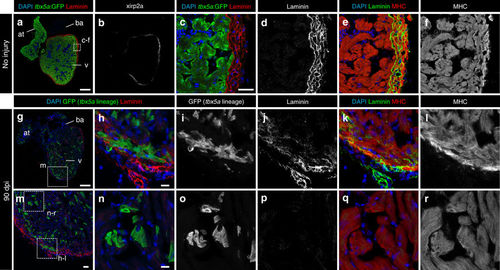
Trabecular tbx5a-derived cardiomyocytes within the cortical myocardium express the cortical marker Xirp2a. Immunofluorescence with anti-Xirp2a and GFP and anti-Myosin Heavy Chain (MHC) on ventricle sections. a–f Uninjured adult tbx5a:GFP ventricle. Xirp2a expression was observed in the cortical layer but not in the trabecular layer showing a complementary pattern with tbx5a:GFP as predicted by the RNA-Seq (n = 3/3). g–r Double transgenic tbx5a:mCherry-p2a-CreERT2;ubb:loxP-lacZ-loxP-GFP were treated with 4-Hydroxytamoxifen (4-OHT) from 84 to 72 and 60 to 48 hours before cryoinjury. Hearts were fixed at 90 days postinjury (dpi). GFP+ cells marking the tbx5a lineage within the cortical layer were positive for Xirp2a, whereas GFP+ cells within the trabecular layer did not express this marker (n = 6/6). at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bars, a, g 100 µm, c, m 25 μm, and h, n 10 µm |
|
Trabecular tbx5a-derived cardiomyocytes within the cortical myocardium are surrounded by Laminin. Immunofluorescence with anti-Laminin, anti-GFP, and anti-Myosin Heavy Chain (MHC) on ventricle sections. a–f Uninjured adult tbx5a:GFP ventricle. Laminin expression was observed in the cortical layer but not in the trabecular layer showing a complementary pattern with tbx5a:GFP (n = 3/3). g–r Double transgenic tbx5a:mCherry-p2a-CreERT2;ubb:loxP-lacZ-loxP-GFP adult fish were treated with 4-Hydroxytamoxifen (4-OHT) from 84 to 72 and 60 to 48 hours before cryoinjury. Hearts were fixed at 90 days postinjury (dpi). GFP+ cells marking the tbx5a lineage within the cortical layer were surrounded by Laminin staining (n = 6/6). at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bars, a, g 100 µm, c, m 25 μm, and h, n 10 µm |
|
Summary of the contribution of tbx5a-positive and -negative myocardium during heart development and regeneration. a Identification of tbx5a-expressing and tbx5a-derived cells in the zebrafish heart. Red, tbx5a+ cells; green, tbx5a-derived cells not expressing tbx5a; yellow, tbx5a-expressing cells derived from embryonic tbx5a+ cells. b Replacement of the embryonic first heart field myocardium with second heart field progenitors. Blue, ventricular cardiomyocytes; red, tbx5a-derived ventricular cardiomyocytes; dotted red, ablated tbx5a-derived ventricular cardiomyocytes. c Contribution of tbx5a-derived cells during heart regeneration in the zebrafish. Red, tbx5a+ cells; green, tbx5a-derived cells not expressing tbx5a; yellow, tbx5a-expressing cells derived from trabecular adult tbx5a+ cells. 4-OHT, 4-Hydroxytamoxifen; at, atrium; Mtz, Metronidazole; v,ventricle |
|
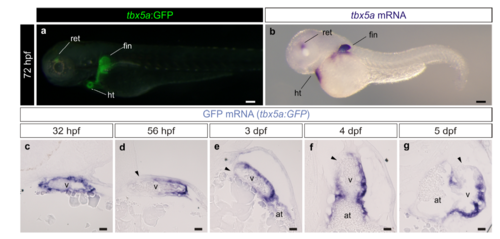
Characterization of the tbx5a:GFP reporter line. a Lateral view of a tbx5a:GFP larvae at 3 days postfertilisation. A merged fluorescent and brightfield image is shown. b mRNA in situ hybridization (ISH) with a tbx5a antisense riboprobe on the same staged larvae as the one shown in a (n=7/7). c–g gfp mRNA on embryonic heart sections of tbx5a:GFP fish at different stages of development. The black arrowheads point to the tbx5a:GFP- area of the ventricle. at, atrium; dpf, days postfertilisation; hpf, hours postfertilisation; ht, heart tube; ret, retina; v, ventricle. Scale bars, a, b 100 μm and c, g 25 μm |
|
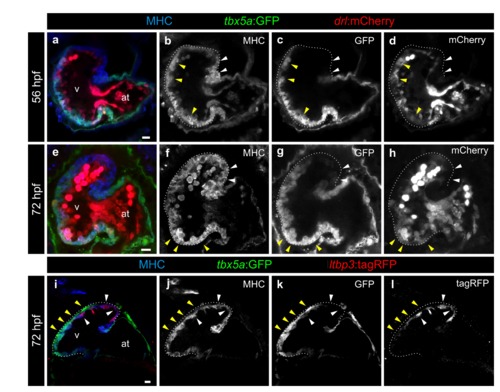
The pattern of tbx5a:GFP expression is similar to that of drl:mCherry and complementary to ltbp3:mCherry. a–h Confocal optical sections of 56 (n=5/5) and 72 (n=7/7) hours postfertilisation (hpf) tbx5a:GFP;drl:mCherry double transgenic zebrafish larvae. GFP (green) labels tbx5a+ cells, mCherry (red) drl+ cells and anti-Myosin Heavy Chain (MHC) immunofluorescence labels all cardiomyocytes. The ventricle is outlined with dotted lines. The tbx5a:GFP- and drl:mCherry- distal ventricle is marked with white arrowheads, while the yellow arrowheads point to the domains positive for both markers. Note that drl:mCherry is also expressed in endocardial cells and red blood cells in the lumen of the heart. i–l Confocal optical sections of 72 hpf hearts from tbx5a:GFP embryos injected with ltbp3:TagRFP-2A-Cre at the one cell stage. Shown is a representative heart out of 8 larvae. GFP labels tbx5a+ cells, mCherry ltbp3+ cells, and MHC all cardiomyocytes. The yellow arrowheads point to tbx5a:GFP+ cells that are ltbp3:mCherry- while the white arrowhead points to a ltbp3:mCherry+ cells within the tbx5a:GFP- domain. at, atrium; v, ventricle. Scale bars, 10 μm |
|
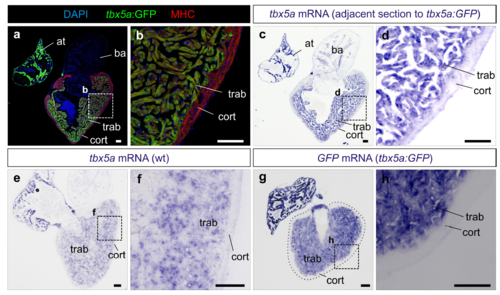
The transgenic tbx5a:GFP reporter line recapitulates the expression of endogenous tbx5a. a,b Sagittal section through an adult tbx5a:GFP zebrafish heart immunostained for GFP and Myosin Heavy Chain (MHC), (n=8/8). c,d mRNA in situ hybridization (ISH) with a tbx5a antisense riboprobe on the adjacent section shown in a and b. Note expression of GFP and tbx5a mRNA in the trabecular myocardium and their absence of expression in the cortical myocardium (n=8/8). b and d are zoomed views of boxed areas in a and c. e,f tbx5a ISH on a sagittal heart section of an AB wildtype adult zebrafish (n=5). g,h GFP ISH on a sagittal heart section of tbx5a:GFP zebrafish (n=4). at, atrium; ba, bulbus arteriosus; cort, cortical layer; trab, trabecular layer; wt, wildtype. Scale bars, 100 μm |
|
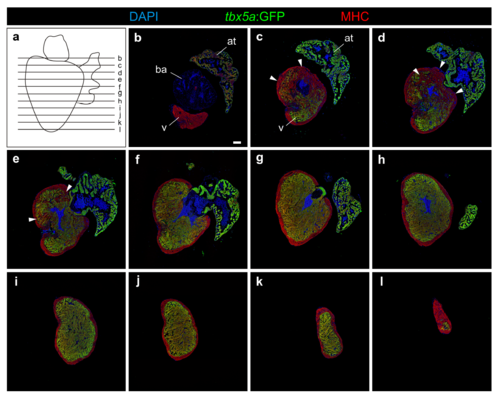
Serial sections of a tbx5a:GFP transgenic adult heart. a Scheme showing the direction used for sectioning the heart and domain represented in images b–l. b–l Serial sections of a tbx5a:GFP transgenic adult heart immunostained for GFP (tbx5a+ cells, green) and Myosin Heavy Chain (MHC) (cardiomyocytes, red). Nuclei are counterstained with DAPI. Arrowhead marks the tbx5a:GFP negative region in the basal ventricle (n=13/13). at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bar, 100 μm |
|
Fate mapping of embryonic tbx5a-derived cells. a–d Optical section through a tbx5a:CreERT2;ubb:loxP-GFP-loxP-mCherry heart at 4 days postfertilisation (dpf) treated with 4-Hydroxytamoxifen (4-OHT) as shown in a revealing efficient recombination in ventricular and atrial cardiomyocytes (mCherry, red). Shown is a section through the atrioventricular canal region. The distal tbx5a- region is not visible in this section. See Supplementary Movie 5 for a full z-stack visualization. at, atrium; hpf, hours postfertilisation; v, ventricle. Scale bars, 10 μm |
|
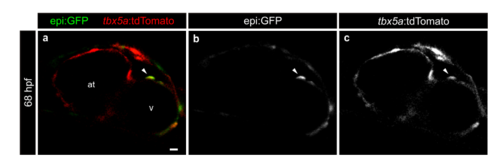
tbx5a:tdTomato colocalizes with an epicardial marker transgenic line. Shown is an optical section of a heart at 68 hours postfertilisation (hpf) from a double transgenic tbx5a:tdTomatocn13; Et(-26.5Hsa.WT1-gata2:EGFP)cn12 animal. Arrowhead marks a double positive epicardial cells on the ventricle (observed in 6 out of 7 larvae). at, atrium; v, ventricle. Scale bar, 10 μm |
|
Fate mapping of embryonic tbx5a-derived cells on transverse sections of adult hearts. a Overview of the experimental setup. b Scheme showing the sectioning orientation through the heart and the location of the individual sections shown in the figure. c–q Immunofluorescence staining of adult heart sections recombined as in a. Shown are merged channels for GFP (green), mCherry (red) and anti-MHC staining (blue). Arrowheads point to the negative basal domain in the ventricle (n=2/2). at, atrium; v, ventricle. Scale bar, 100 μm |
|
Fate mapping of embryonic tbx5a-derived cells on sagittal sections of adult hearts. a Overview of the experimental setup. b Scheme showing the sectioning orientation through the heart and the location of the individual sections shown in the figure. c–i Immunofluorescence staining of adult heart sections recombined as in a. Shown are merged channels for GFP (green), mCherry (red) and anti-MHC staining (blue). Arrowheads point to the negative basal domain in the ventricle n=3/3. Scale bar, 100 μm |
|
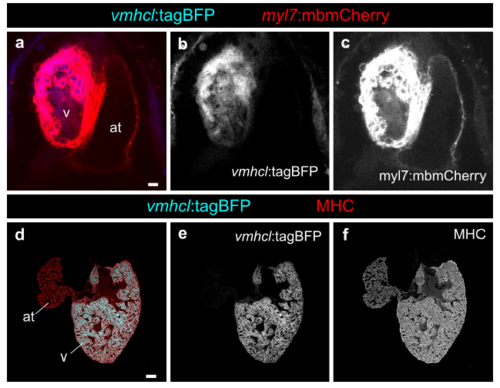
Characterization of the vmhcl promoter to drive expression specifically in the cardiac ventricle. a–c Heart from larvae at 4 days postfertilisation (dpf) showing that the vmhcl:loxP-tagBFPloxP- mCherry-NTR line is specific for ventricular cardiomyocytes (n=10/10). d–f Specificity and expression is maintained in the adult (n=9/9). at, atrium; v, ventricle. Scale bars, a 10 μm, d 100 μm |
|
Assessment of efficient ablation of tbx5a-derived cardiomyocytes in individualized fish. a tbx5a+ ventricular cardiomyocytes were genetically ablated in tbx5a:CreERT2;vmhcl:loxP-tagBFP-loxP-mCherry-NTR double transgenic zebrafish by administration of Metronidazole (Mtz). Recombination was induced by administration of 4-Hydroxytamoxifen (4-OHT) at 1 and 2 days postfertilisation (dpf). At 4 dpf, larvae where imaged under a binocular scope. b–o Images show lateral views of the head and heart region. The lens is red (gamma-crystallin transgenic reporter), the cardiac ventricle is red (mCherry+ tbx5a-derived ventricular cardiomyocytes) and blue (tbx5a- cardiomyocytes). Fish were individually treated with Mtz from 4 to 7 dpf. At 7 dpf, 3 days after initiation of Mtz treatment, each larva was imaged again. At this stage, tagBFP expression is visible and expanded but mCherry is no longer detected in most larvae. FHF, first heart field; SHF, second heart field. Scale bars, 100 μm |
|
Genetic ablation of tbx5a-derived ventricular cardiomyocytes in individualized fish. a tbx5a+ ventricular cardiomyocytes were genetically ablated in tbx5a:CreERT2;vmhcl:loxP-tagBFP-loxP-mCherry-NTR double transgenic zebrafish. Recombination was induced by administration of 4- Hydroxytamoxifen (4-OHT). Fish were individualised, imaged at 4 days postfertilisation (dpf) and cell ablation was induced by administration of Metronidazole (Mtz) from 4 to 7 dpf. b,f Maximum projection of confocal z-stacks of 4 dpf embryos. c–e, g–i Hearts dissected from the same fish at 42 dpf. at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bars, b, f 25 μm, c, g 100 μm |
|
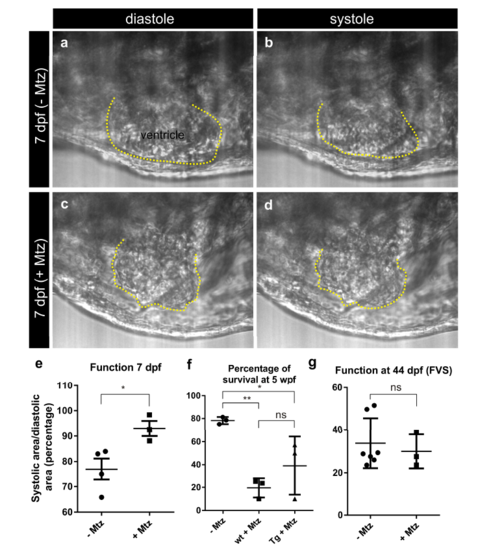
Cardiac performance after ablation of tbx5a-derived cells. tbx5a+ ventricular cardiomyocytes were genetically ablated in tbx5a:CreERT2;vmhcl:loxPtagBFP- loxP-mCherry-NTR double transgenic zebrafish. Recombination was induced by administration of 4-Hydroxytamoxifen (4-OHT) at 1 and 2 days postfertilisation. Animals were divided into two groups. Cell ablation was induced in one group by administration of Metronidazole (Mtz) from 4 to 7 dpf. Videos were acquired at 7 dpf, immediately after the last Mtz treatment. a–d are still images from the videos from two Mtz-treated (from a total of 4) and two non-treated (from a total of 3) animals. Shown are lateral views of the heart, the head is to the left. The ventricle is outlined in yellow. Note the irregular shape and overall smaller area in Mtz-treated hearts. e The maximum (diastolic) and minimum (systolic) ventricular area was measured to determine ventricular function. P= 0.0348 by a two-tailed t-test. Deficient contraction was detected upon Mtz treatment. f Percentage of survival at 5 weeks post fertilization (wpf); mean±s.d **P<0.01; *P<0.05; ns, nonsignificant by one-way ANOVA followed by Tukey’s multiple comparisons test. Each point represents the survival percentage of a group comprising 14-22 fish each. –Mtz: same stages control fish; sib+Mtz= tbx5a:CreERT2 and vmhcl:loxP-tagBFP-loxPmCherry- NTR single transgenic siblings; Tg+Mtz= tbx5a:CreERT2;vmhcl:loxP-tagBFPloxP- mCherry-NTR double transgenic fish. g Assessment of ventricular Fractional Volume Shortening (FVS) by echocardiography at 44 dpf. Shown are individual measurements as well as mean±s.d; ns, P = 0.6262 by two-tailed unpaired t-test; n=7 for – Mtz group and n=3 for + Mtz group |
|
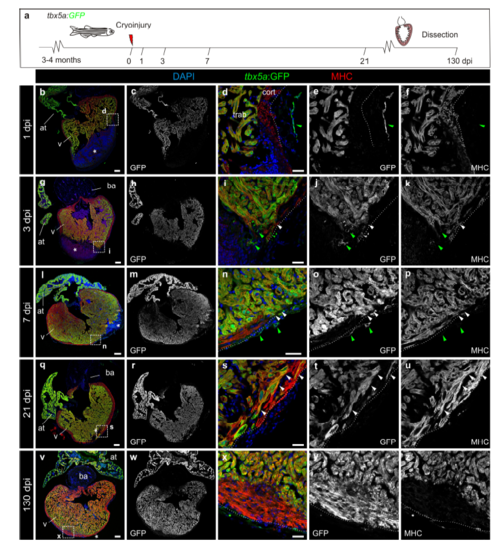
Expression of tbx5a:GFP during adult heart regeneration. a, Illustration of the experimental setup. b–z Sagittal sections through tbx5a:GFP hearts at 1 (b–f n=4/4), 3 (g–k n=4/5), 7 (l–p n=3/3), 21 (q–u n=4/4) and 130 (v–z n=4/4) days post injury (dpi). Sections were immunostained with anti-GFP (green) and anti-Myosin Heavy Chain (MHC; red). Nuclei are counterstained with DAPI (blue). Asterisks indicates the injury area, dotted line marks the outer border of the cortical layer. tbx5a:GFP expression is limited to the trabecular myocardium. Few myosin-negative tbx5a:GFP+ cells are found within the epicardial layer (arrowhead). At 130 dpi, a tbx5a:GFP- thickened cortical myocardium covers a tbx5a:GFP+ trabecular myocardium at the injury site (v–z). White arrowheads point to tbx5a:GFP+ cardiomyocytes; green arrowheads label tbx5a:GFP+ noncardiomyocytes. Scale bars, b, g, l, q, v 100 μm, d, i, n, s, x 25 μm |
|
Trabecular cardiomyocyte lineage tracing control experiments. a–f tbx5a:mCherryp-2a-CreERT2 was crossed into ubb:loxP-lacZ-STOPloxP- GFP. Fish were treated with 4-Hydroxytamoxifen (4-OHT) at days 3 and 2 before fixation. Arrowhead points to one of the 2 cells GFP+ cells identified in 1 out of 6 hearts. g– q loxP sites in tbx5a:Cherryp-p2a-CreERT2;ubb:loxP-lacZ-STOP-loxP-GFP do not recombine in the absence of 4-OHT. g tbx5a:Cherry-p2A-CreERT2 was crossed into ubb:loxP-lacZ-STOP-loxP-GFP. Fish were cryoinjured but not treated with 4-OHT. h–l Heart before injury. No recombined cells were observed (n = 4/4). m–q Hearts were injured and dissected at 90 days postinjury (dpi). No GFP+ recombined cells were visible at the regenerated area (n= 3/3). r–w Recombination is not due to the persistence of 4-OHT in the adult fish beyond cryoinjury. r tbx5a:mCherry-p2a-CreERT2 was crossed into ubb:loxPlacZ- STOP-loxP-GFP. Fish were treated with 4-OHT during days 11 to 10 before the injury. s–w Contribution of the GFP+ trabeculae to the new compact layer and tbx5a:mCherry switch off in these cells can be observed (n=3/3). at, atrium; v, ventricle. Scale bars, b, h, m 100 μm, c, i, j, n, o, t, u 25 μm |
|
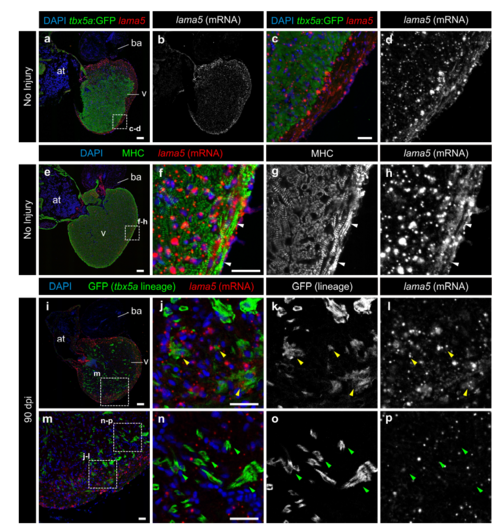
tbx5a-derived cardiomyocytes within the cortical layer are lama5 positive. lama5 RNAScope in situ detection followed by GFP and Myosin Heavy Chain (MHC) immunofluorescence on sagittal sections of tbx5a:GFP (a–h) or tbx5a:mCherryp-2a-CreERT2;ubb:loxP-lacZ-loxP-GFP double transgenic animals, recombined before injury and fixed at 90 days postinjury (dpi) (i-p). Nuclei are counterstained with DAPI. a–d Uninjured heart. tbx5a:GFP (green) does not co-localize with lama5 (red). lama5 is expressed at higher and more homogenous levels in the cortical layer (n=4/4). e–h Uninjured heart. anti-MHC marks cardiomyocytes (green). In the cortical layer, regions of lama5/MHC co-localization were detected (white arrowheads) (n=4/4). i–p 90 dpi regenerated hearts. tbx5a:mCherryp-2a-CreERT2;ubb:loxP-lacZ-loxPGFP were treated with two 12 hours pulses of 4-Hydroxytamoxifen (4-OHT) at 6 and 7 days before the injury. GFP+ cells marking the tbx5a lineage within the cortical layer were positive for lama5 (yellow arrowheads); double positive cells were observed in 11 out of 11 hearts. GFP+ cells within the trabecular layer were negative for that marker (green arrowheads; n=11/11). at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bars, a, e, i 100 μm, c, f, j, n 25 μm |
|
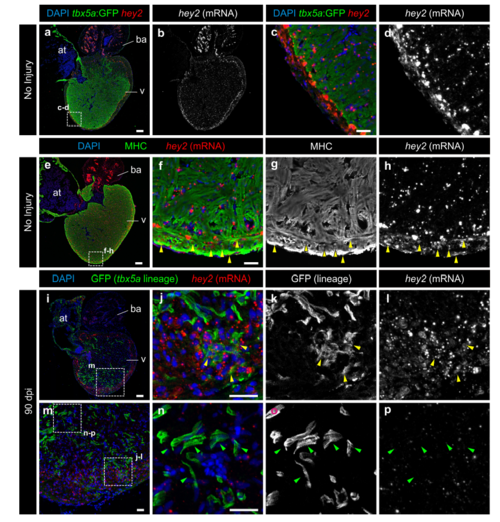
tbx5a-derived cardiomyocytes within the cortical layer are hey2 positive. GFP immunofluorescence and hey2 RNAScope in situ detection on sagittal sections of tbx5a:GFP (a–h) or tbx5a:mCherryp-2a-CreERT2;ubb:loxP-lacZ-loxP-GFP double transgenic animals, recombined before injury and fixed at 90 days postinjury (dpi) (i–p). Nuclei are counterstained with DAPI. a–d Uninjured heart. tbx5a:GFP (green) does not co-localize with hey2 (red). Hey2 is expressed at higher and more homogenous levels in the cortical layer. e–h Uninjured heart. anti-Myosin Heavy Chain (MHC) marks cardiomyocytes (green). In the cortical layer, regions of hey2/MHC co-localisation were detected (yellow arrowheads; n=3/4). i–p 90 dpi regenerated hearts. tbx5a:mCherryp-2aCreER T2;ubb:loxP-lacZ-loxP-GFP were treated with two 12 hours pulses of 4-OHT at 6 and 7 days before the injury. GFP+ cells marking the tbx5a lineage within the cortical layer were positive for hey2 (yellow arrowheads, hey2+/GFP+ cells observed in 5 out of 6 hearts. GFP+ cells within the trabecular layer were negative for hey2 (green arrowheads; n=6/6). at, atrium; ba, bulbus arteriosus; v, ventricle. Scale bars, a, e, i 100 μm, c, f, j, n 25 μm |
|
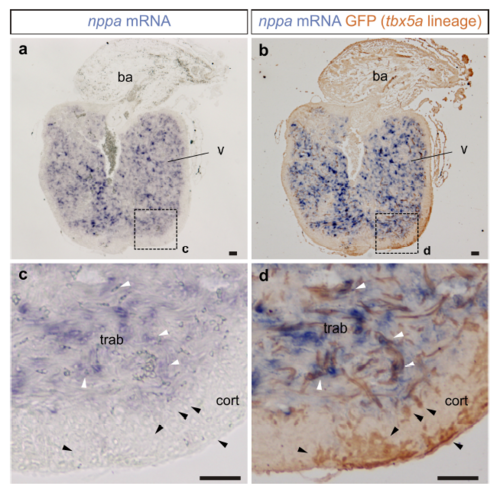
tbx5a-derived cardiomyocytes within the cortical layer are nppa negative. nppa mRNA in situ hybridisation (a) on sagittal sections of tbx5a:mCherryp-2a-CreERT2;ubb:loxP-lacZ-loxP-GFP double transgenic animals, recombined before injury and fixed at 90 days postinjury (dpi) followed by GFP immunofluorescence (b) nppa is expressed in the trabecular layer (white arrowheads). In the cortical region, tbx5a-derived GFP+ cells are visible, which are not positive for the trabecular marker nppa (black arrowheads, n=7/7). c and d are a zoomed with of boxed areas in a and b. ba, bulbus arteriosus; cort, cortical layer; trab, trabecular layer; v, ventricle. Scale bars, 50 μm |