- Title
-
Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish
- Authors
- Harrison, M.R., Bussmann, J., Huang, Y., Zhao, L., Osorio, A., Burns, C.G., Burns, C.E., Sucov, H.M., Siekmann, A.F., Lien, C.L.
- Source
- Full text @ Dev. Cell
|
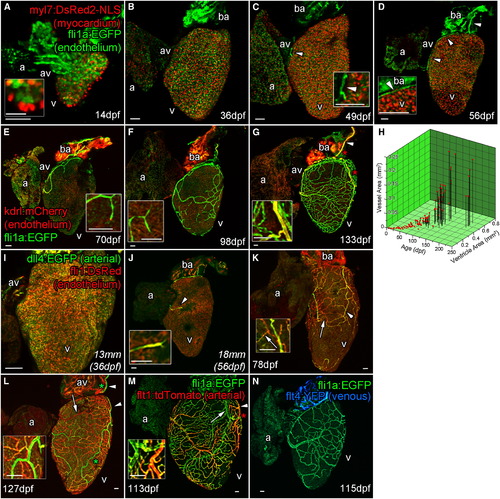
Zebrafish Coronary Vessels Develop during Juvenile Stages and Respond to Increased Heart Size (A–G) Confocal images of whole larval, juvenile and adult double transgenic hearts, Tg(fli1a:EGFP; myl7:DsRed2-NLS (A–D) and Tg(fli1a:EGFP; kdrl:mCherry) (E–G). flila:EGFP-positive endothelial (endocardial) cells are visible only on the inside of the myocardium (labeled with myl7:DsRed2-NLS) and bulbous arteriosus after hatching (A and inset 14 dpf; and B, 36 dpf). Five to seven weeks later, endothelial cells are found on the surface of the heart (C, 49 dpf, arrowhead and inset), forming a vessel connection between the gills and AV-boundary after 1 month (D, 56 dpf, arrowheads and inset). The major vessel over the bulbous arteriosus expresses high levels of kdrl:mCherry (marked by arrowhead in G). Branches from these early vessels appear to progressively cover and interconnect over the juvenile heart into adulthood; however, the majority express low kdrl:mCherry levels (E, 70 dpf to G, 133 dpf, insets). (H and I) The emergence and coverage of these vessels is stochastic, but correlates strongly with age and the area of the ventricle (H). Endocardial cells prior to the emergence of vessels express arterial marker dll4:EGFP (I). (J and K) Emerged endothelial cells on the ventricle surface also have an arterial identity (J, arrowhead and inset), which is selectively downregulated in some vessels as they form (K, arrow). (L and M) A subset of vessels maintain dll4:EGFP (L, arrowhead) and express flt1:tdTomato confirming they are arteries (M, arrowhead). (N) Vessels that downregulate arterial makers appear to not progress to full venous identity as flt4:YFP expression is not observed at significant levels. a, atrium; av, AV canal; v, ventricle. Zebrafish age listed as dpf or italicized total body lengths with equivalent age in brackets when zebrafish were raised under non-standard conditions. artery. Scale bars represent 50 µm except for that in (A, inset), which is 10 µm. |
|
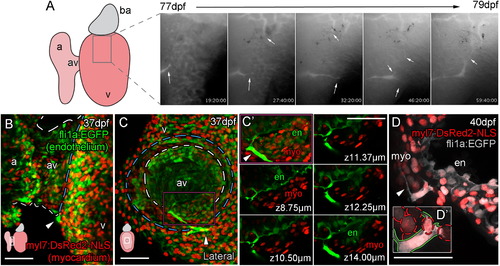
Vessels Are Formed from Angiogenic Sprouts of Cells Emerging from the AV Canal (A–D′) Live imaging stills show the angiogenic migration of the endothelial cells that migrate over the ventricle surface and form interconnections between forming vessels (A; angiogenic sprouts marked by arrows). The first emerging endothelial cells are visible on the surface of the ventricle at the juncture of the atrium and ventricle (arrowheads, B, ventral view; C, lateral view of the same heart, diagram denotes heart orientation). Cells at this point have a direct connection to the endocardium (C, dashed white line demarks AV canal, blue line marks invagination of the ventricular myocardium; C′, higher magnification of region demarcated by purple box with select individual planes in z, labeled with distance from the first plane of projection). Individual endothelial cells span the myocardium (D; D′, higher magnification with red dashed lines demarcating myocardial nuclei, green line demarcates endothelial cell) and then project filopodia over the ventricle surface (D, arrowhead). Scale bars represent 50 µm. |
|
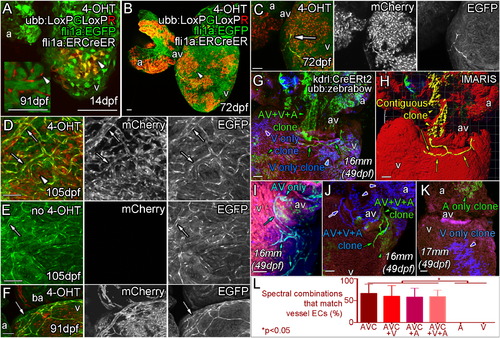
Angiogenic Coronary Endothelial Cells Are Derived from a Subpopulation of the Endocardium Triple transgenic fish, Tg (ubb:LoxPEGFPLoxPmCherry (ubb:LoxPGLoxPR); fli1aep:ERt2CreERt2 (fli1a:ERCreER); fli1a:EGFP) were treated with 4-OHT during embryogenesis (0–5 dpf) to label the endocardium as mCherry positive clonal patches (arrowheads) and imaged at 14 dpf (A), 72 dpf (B and C), 91 dpf (F) and 105 dpf (D). (E) No 4-OHT control. (A, inset) 91 dpf section with no fli1a:EGFP showing mCherry expression in endocardial cells lining the trabecular myocardium. Emerging endothelial cells (fli1a:EGFP-positive, bright green) are also mCherry-positive (C and D, arrow, labeled endothelial cells; arrowhead, labeled endocardial cells). The majority of subsequently formed vessels are found to be similarly labeled in adult zebrafish treated with 4-OHT as embryos (D, arrows), but not in those that were not exposed to 4-OHT during embryogenesis (E, arrow). Unlabeled vessels (arrowhead) have been observed on hearts with labeled vasculature consistent with the hypothesis that multiple cells give rise to vessels by an angiogenic process (F, arrow). Clonal analysis with multispetral fluorescent proteins (ubb:Zebrabow; ubb:Lox2272-LoxP-RFP-Lox2272-CFP-LoxP-YFP) is used to follow the fate of restrictedly labeled endocardial cells (G–L). Image of the ventral side of the AV canal, with ventricle below and atrium above showing labeling of the endocardial cells as a single green clone (G, green arrowhead), which gives rise to the forming vessel on the ventricle surface (G, green arrows). Part of this green clone is contiguous with the surface endothelial cells, extending through the myocardium to the AV canal and atrium (H, yellow). Analysis of multiple clones, including AV endocardium specific clones (I), shows a clonal relationship between AV endocardial cells and endothelial cells on the surface (G–J). Forming vessels can be derived from more than one clone, but always share the same labeling with the AV canal (J). Non-AV clones are found not to give rise to surface endothelium (K). Quantification of the endocardial spectral combinations found in different heart regions that match those of the vessel endothelium (L, p < 0.05 ANOVA/Tukey, ± SEM). Zebrafish age listed as dpf or italicized total body lengths with equivalent age in brackets when zebrafish were raised under non-standard conditions. AVC, atrioventricular canal; A, atrium; V, ventricle. Scale bars represent 50 µm. |
|
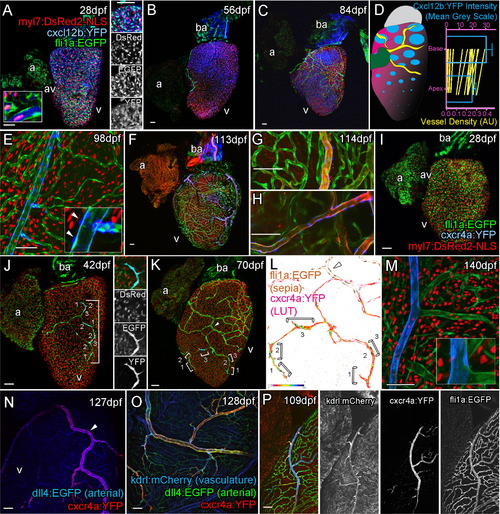
Cxcr4a-Cxcl12b Expression during Coronary Vessel Formation Confocal images of transgenic fish Tg(myl7:DsRed2-NLS; fli1:EGFP; cxcl12b:YFP) (A–C, E), Tg(kdrl:mCherry; fli1:EGFP; cxcl12b:YFP) (F–H), Tg(myl7:DsRed2-NLS; fli1:EGFP; cxcr4a:YFP) (I–M), Tg(dll4:EGFP; cxcr4a:YFP) (N), Tg(dll4:EGFP; cxcr4a:YFP; kdrl:mCherry) (O), and Tg(kdrl:mCherry; fli1:EGFP; cxcr4a:YFP) (P). Ventricular, but not atrial cardiomyocytes express cxcl12b:YFP prior to and during vessel formation (A, 28 dpf; B, 56 dpf; C, 77 dpf). cxcl12b:YFP labeling is found within the cytoplasm of cells containing a myl7-positive nuclei (red) and flanked by fli1a-positive (green) endothelial cells (A, inset, from confocal section, side image). Levels of cxcl12b:YFP expression vary over the ventricle and between hearts, but are generally higher toward the base (A–D). Quantification of cxcl12b:YFP intensity (blue, normalized mean 14 dpf to 84 dpf, ± SEM) and vessel density (green, mean values of individual time points 7 dpf to 420 dpf) between base and apex of ventricular myocardium (D), both p < 0.0001 (Wilcoxon signed rank test on paired values). In addition, expression is observed on the surface of the bulbous arteriosus (ba, A–C, F). cxcl12b:YFP expression is downregulated following establishment of the vessel network in cardiomyocytes, but is later expressed in the mural cells of mature vessels (E, inset arrowhead). cxcl12b:YFP-expressing mural cells surround large arterial vessels (F–H, blue), which express high levels of kdrl:mCherry (F–H, red). cxcr4a:YFP is not expressed prior to the emergence of endothelial cells on the surface of the ventricle (I, 28 dpf). Endothelial cells of angiogenic sprouts express cxcr4a:YFP as they migrate over the surface of the myocardium (J, 42 dpf, image showing tip cell; K, 70 dpf; brackets label cells displaying active migration morphology; L, intensity of cxcr4a:YFP in endothelial cells). The majority of vessels do not express cxcr4a:YFP after their formation (K–P). Larger arterial vessels do maintain cxcr4a:YFP expression, which overlaps with high levels of kdrl:mCherry and dll4:EGFP expression in these vessels (N–P). Scale bars represent 50 µm. |
|
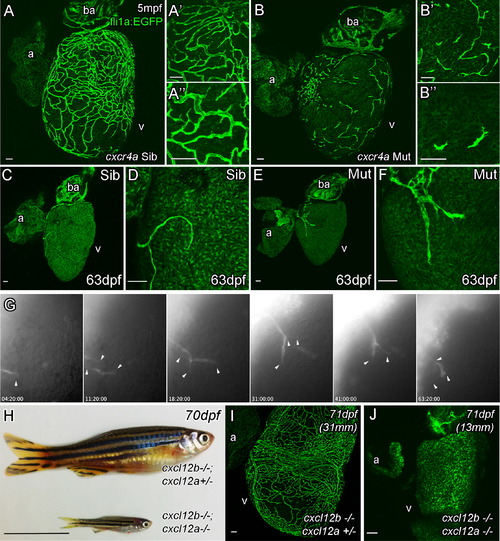
Cxcr4a and Cxcl12 Are Required for Coronary Vessel Formation Endothelial cells migrate over the surface of the heart, but fail to form a vascular network in adult cxcr4a mutant fish (A, Sibling control and B, Mutant). As cxcr4a mutant endothelial cells emerge onto the surface of the heart in juvenile zebrafish, they have an uncharacteristic shape appearing truncated and highly spiculated (C–F). Movie stills show abnormal migration of endothelial cells in the mutant (G). Unlike wild-type cells, cxcr4a mutant cells project and retract multiple processes in different directions while migrating over the ventricle (arrowheads). They often migrate individually in different changing vectors and fail to make and maintain connections between each other. cxcl12a; cxcl12b double mutants show general retardation in growth in comparison with siblings raised under the same (optimized) husbandry conditions (H). Unlike their siblings, no endothelial cells are observed on the surface of heart of cxcl12a/b double mutant fish (I, sibling; J, cxcl12a; cxcl12b double mutant). Italicized age indicates zebrafish are raised using optimized husbandry, body length in brackets. Scale bars represent 50 µm, except in (H), which is 10 mm. |
|
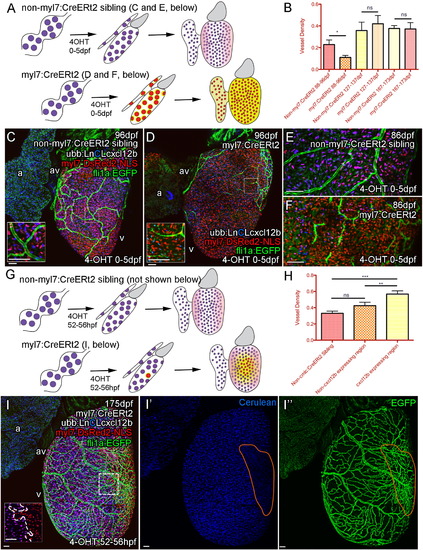
Cxcl12b Likely Provides Positional Information for Coronary Endothelial Cells (A–E) Uniform overexpression of Cxcl12b in quadruple transgenic fish Tg(Ubb:LoxPH2B-Cerulean-LoxP-cxcl12b(ubb:LnCLcxcl12b); myl7:DsRed2-NLS; fli1a:EGFP; myl7:CreERt2). Schematic illustration of uniform overexpression of Cxcl12b. Prolonged treatment with 4OHT between 0 and 5 dpf resulted in efficient removal of the floxed H2B-Cerulean (blue) and expression of cxcl12b (yellow) in myl7-positive cardiomyocytes (red) such that any endogenous Cxcl12b signaling pattern may be obscured (pink) (A). Such overexpression of cxcl12b in cardiomyocytes also resulted in an initial disruption of vessel formation (B, quantification of vessel density, p < 0.05 t test, ± SEM; C, non-myl7:CreERt2 sibling control; D, cxcl12b overexpression) with endothelial cells becoming elongated and appearing spiculated (C and D, inset, E [control], and F [overexpression]). (G–I) Clonal overexpression of Cxcl12b in quadruple transgenic fish Tg(Ubb:LoxPH3B-Cerulean-LoxP-cxcl12b (ubb:LnCLcxcl12b); myl7:DsRed2-NLS; fli1a:EGFP; myl7:CreERt2). A 4-hr pulse of 4OHT results in partial activation of the myl7:CreERt2 transgene prior to cardiomyocyte clonal expansion. cxcl12b is then expressed from a localized source in adult zebrafish (G, I; inset from box; boundary between exogenous-cxcl12b expressing [DsRed-NLS positive] and non-expressing [H2B-Cerulean and DsRed-NLS double positive] cardiomyocytes is marked with a dashed line). There is an increase in density of vessels over and proximal to the region of cxcl12b expressing cardiomyocytes (H, I, I′, and I′′, region of cxcl12b-expressing (Cerulean-negative) cardiomyocytes demarcated by orange). (H) Quantification of vessel density. Representative image of clonal overexpression (I, I′, and I′′) with region of cxcl12b-expressing (Cerulean-negative) cardiomyocytes demarcated by orange. p < 0.01; p < 0.0001 t test. Scale bars represent 50 µm. |
|
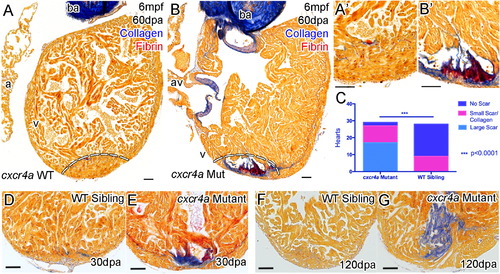
cxcr4a Mutant Hearts Fail to Regenerate following Resection of the Ventricle Apex Zebrafish with functional Cxcr4a-Cxcl12b signaling and normal coronary vessel development completely regenerate resected ventricle tissue after 60 days (A, dashed line represents amputation plane, and A′). Adult cxcr4a mutants with disrupted coronary vessel development fail to regenerate the resected tissue resulting in the deposition and persistence of collagen (blue) and fibrin (red) (B, B′, E, and G). Quantification of scar formation at 60 dpa (C). cxcr4 mutant zebrafish have significantly more collagen deposition and scar formation compared to the siblings (p < 0.0001, chi-square). Scar formation is observed by 30 dpa (D and E) and persists beyond 120 dpa (F and G) with no apparent effect on survival. Scale bars represent 50 µm. |
|
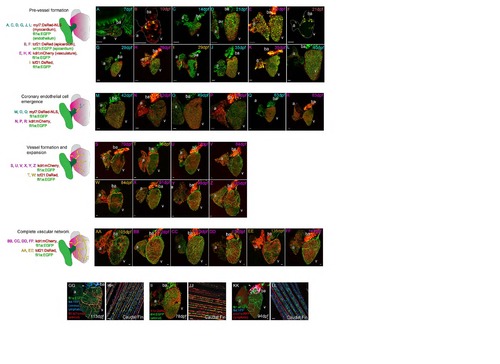
Postembryonic zebrafish coronary vessel development. (Related to Figure 1) Four different transgenic lines were used in different combinations to characterize the development of coronary vessels in zebrafish from 7dpf to 140dpf; fli1a:EGFP; myl7:DsRed2-NLS (labeled blue: A, C, D, G, J, L, M, O and Q), wt1b:EGFP; tcf21:DsRed (labeled maroon: B and F), fli1a:EGFP; kdrl:mCherry (labeled pink: E, H, K, N, P, R, S, U, V, X, Y, Z, BB, CC, DD, FF), fli1a:EGFP; tcf21:DsRed (labeled orange: I, T, W, AA, EE). Pre-vessel formation (7 to 40dpf). After hatching the heart is composed of three layers, the fli1a:EGFP endocardium (A), cmlc:nRFP positive myocardium (A) and the epicardium partially labeled with wt1b:EGFP and tcf21:DsRed (B). The heart increases in size dramatically through the first six weeks of life (A-L) during which time the myocardium expands and the tcf21-poisitive epicardium partially covers the surface of both the atrium (a) and ventricle (v) (B, F, I). The bulbus arteriosus (ba) strongly expresses kdrl:mCherry as does the endocardium (E, H, K), which is visible through the myocardium. There are no endothelial cells (fli1a:EGFP) on the surface of the myocardium (cmlc:nRFP, A, C, D, G, J and L). Coronary endothelial cell emergence (42 to 63dpf). Following this expansion in the six weeks after fertilization, endothelial cells are found on the surface of the heart and the coronary vasculature begins to form (M-R). These endothelial cells are found on the outside of the myocardium (cmlc:nRFP, M, O, Q) and express kdrl:mCherry (N, P, R). They are first observed at the juncture of the atrium and ventricle (M-O) and then connected from this point over the base of the ventricle (P-R). Vessel formation and expansion (70 to 105dpf). The heart continues to grow into adulthood as it does so the vasculature continues to form over the surface of the ventricle, but not the atrium (S-Z). Endothelial cells remain connected to existing sprouts or vessels during this process (S-Z) and the heart becomes increasingly covered by tcf21-possitive epicardial cells as it proceeds (T and W). Complete vascular network (105 to 140dpf). By 4 months post-fertilization the intricate network of vessels is formed, the pattern and density of vessels varies between adult zebrafish, but vessels are only observed on the ventricle of the heart (AA-FF). A subset of endothelial cells of the coronary vasculature network express arterial markers flt1:tdtomato (GG) and dll4:EGFP (II), but none of the vasculature endothelial cells express venous marker flt4:YFP (GG and KK) nor lymphatic marker prox1:RFP (KK) at this stage. Expression of both prox1a:RFP and flt4:YFP is restricted to endothelium partially covering the BA (GG and KK, arrowheads). All four markers were found to be expressed in the adult fin of the same fish (flt1:tdtomato and flt4:YFP, HH; dll4:EGFP, JJ; prox1a:RFP and flt4:YFP, LL). Asterisk denotes artery. Scale bars, 50 µm. |
|
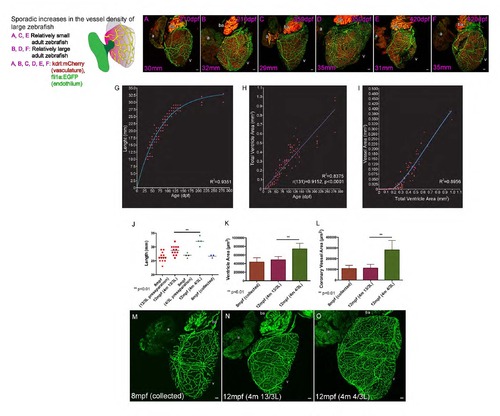
Coronary vasculature remains responsive to increases in heart size and output requirement. (Related to Figure 1) Sporadic increases in the vessel density of large zebrafish (210 to 420dpf). Although formed and stable in many zebrafish the coronary vasculature remains capable of expanding in size and density with volumetric increases in heart size in older zebrafish (A-F). A proportion of zebrafish can continue to grow larger than their sibling tank mates and show increases in heart size and coronary vessel density (210dpf, A and B; 350dpf, C and D; 420dpf E and F; body length between 29 and 35mm labeled bottom left of each panel). The developmental timing, organization and extensiveness of the coronary vessel network are slightly variable, but highly correlative with fish size, age and the size of the heart ventricle (G-I). Graphs demonstrating the one-phase exponential correlation between zebrafish length and age (G, coefficient of determination (R2)), linear correlation between heart ventricle size and age (H, Pearson correlation coefficient (r)) and the two-phase exponential correlation between heart ventricle size and the area covered by endothelial cells on the surface of the heart (I). Scale bars, 50 µm. Adult sibling zebrafish are raised and maintained at a high density of 20 fish per 3 liter (L) until 8 mpf and then measured (8 mpf, preseparation) and divided into two groups: one maintained at high density (13/3L) and the other at low density (4/3L) (J). Three of these fish with an average length of 27mm were collected and imaged (8mpf collected, J). Four months later (12mpf), zebrafish from both groups (4m 13/3L and 4/3L) were re-measured. Zebrafish maintained at low density are significantly larger (3mm on average) than those maintained at high density (J, 12mpf 4m 4/3L and 13/3L respectively; **p<0.01, t-test ±SD). Imaging of ventricle area (K) and vessel area (L) in 8 mpf (20/3L, collected), and 12 mpf hearts of fish maintained at high (4m 13/3L) and low (4m 4/3L) density reveals that the ventricle and corresponding coronary vessel area and density are significantly greater in the group of zebrafish maintained at low density (K and L, **p<0.01, t-test ±SD; M, collected preseparation; N, high density; O, low density). Scale bars, 50 µm. |
|
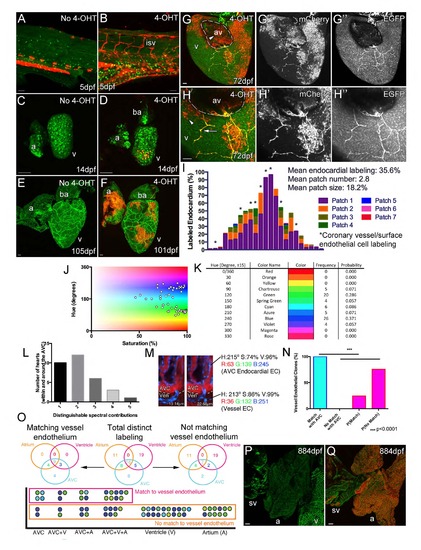
Cell lineage tracing of endocardial cells. (Related to Figure 3) Cre activity is strictly dependent on 4OHT with no labeled endothelial cells observed in non-4OHT treated embryos (A, red autofluorescence from pigment cells), larval (C) or adult fish (E). Treatment of embryos for 5 days in 4-OHT results in clonal endothelial cell labeling in the embryo (B), larval (D) and adult (F) zebrafish. However, such labeling of the endothelial cells does not always result in the labeling of the later developing coronary vessels indicating that only a subset of endocardial cells contribute to the coronary vasculature (F). In addition, labeling of vessels in 4OHT treated hearts that lack any endocardial labeling has not been observed (n=6). Side view of ventricle at the AV boundary (atrium removed) (G and H, arrowhead, labeled endocardium; arrow, labeled surface vessel; G′ and H′ mCherry, G′′ and H′′ EGFP). Labeled endocardial cells in the AV boundary can be seen proximal to similarly labeled migrating endothelial cells on the ventricle surface. I, Quantification of triple transgenic zebrafish hearts (fli1aep:ERt2CreERt2; fli1a:EGFP; ubb:LoxP-EGFP-LoxPmCherry) exposed to 4-OHT during embryogenesis and imaged at 95 to 155dpf, cumulative proportion of endocardium labeled in 1 to 7 patches (defined as a continuous group of cells). Individual hearts along x-axis, hearts with labeled coronary vessels or surface endothelial cells are marked with asterisk (I). To confirm the contribution from the AVC endocardium, recombination of ubb:Zebrabow (ubb:Lox2272-LoxP-RFP-Lox2272-CFP-LoxP-YFP) was induced in the embryonic endocardium by kdrl:CreERt2 to produce a spectrum of colors which are plotted with respect to their hue and saturation (J). There was limited contribution to the spectrum from the red channel due the relatively weak expression of ubb:Zebrabow in endothelial cells and the imaging limitations due to the adjacent RFP-positive myocardium. Despite this, a range of distinguishable colors was detected with varying frequency (K). An average of 2.2 different colors where found in the regions surrounding the AVC and a maximum of 5 spectral labels were distinguishable within a single heart (L). An example of a color specific to the AVC endocardium that matches that of the vessel endothelium on the ventricle surface, there is also a direct connection between these cells (M; relative z position listed for AVC/vessel clone). Even without a full spectrum of color labeling the spectral matching of AVC endocardium with vessel endothelial cells is significantly higher than expected at random (N, Probability of randomly matching colors [P(Match)] is 24.4%, p<0.0001). Positional distribution of distinct spectral contributions shows that many are shared between different heart regions, but only those that label AVC endocardium match the labeling observed in vessel endothelial cells (O). The different spectral contributions are represented as a circle each of which is positioned with respect to their location within the endocardium of the heart (O). Colors that match those of the vessel endothelium on the ventricle surface are grouped together in the pink box; those that do not appear to match are below in the orange box (O). Unlike single-channel analysis, for multispectral analysis only cell spectral contribution is scored and not relative cell positioning (i.e. whether or not cells form a contiguous patch). Vessel density is defined as a ratio of this vessel area to total pixel area of a defined region. The positioning of the emerging sprouts from the AVC endocardium is consistent with the pattern of vascularization observed in older zebrafish hearts, which lack vessels on the surface of the atrium and sinus venous (sv, fli1a:EGFP) (P). Unlike the SV in other organisms studied, the zebrafish SV largely lacks cardiomyocytes (Q, myl7:DsRed2-NLS, red) and has no coronary sinus on its surface (Q, fli1a:EGFP, green; ventricle removed and atrium rotated to reveal SV). Scale bars, 50 µm. isv, intersegmental vessel; a, atrium; v, ventricle; av, AV canal; and sv, sinus venous. |
|
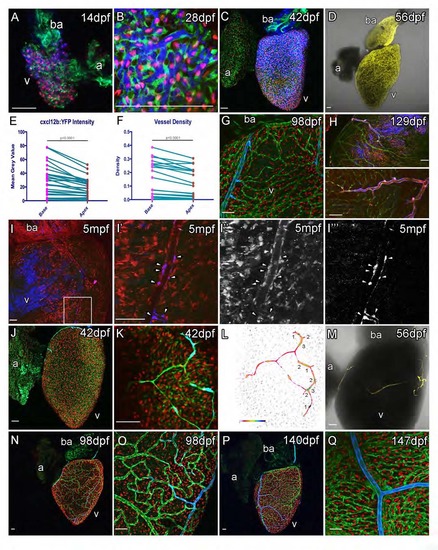
cxcl12b:YFP and cxcr4a:YFP expression during vessel formation. (Related to Figure 4) Prior to the emergence of vessels, cxcl12b:YFP (pseudo colored blue in A, B, C, G, H, I; yellow in D) is detected in ventricular cardiomyocytes that also express myl7:DsRed2-NLS and are flanked by fli1a:EGFP positive endocardial cells (AC). Expression of cxcl12b:YFP is stronger in the base than the apex (E, between 14dpf and 84dpf, n=36, measurements paired within hearts) and coronary vasculature is significantly more dense in the base of the ventricle (F, between 7 and 420dpf, n=153, means of 22 time points are paired), both p<0.0001 (Wilcoxon matched-pairs signed rank test). After the formation of coronary vessels expression is observed in mural cells (G-I). tcf21:CreERt2, ubb:LoxPEGFP- LoxP-mCherry, cxcl12b:YFP embryos are treated with 4OHT to label the embryonic endocardium. mCherry-positive, epicardium (tcf21+) derived cells line the vessels and are cxcl12b:YFP positive (I, box enlarged to I′, I′′ mCherry, I′′′ YFP). Endothelial cells that migrate out over the ventricular cardiomyocytes express cxcr4a:YFP (J, K, L and M). cxcr4a:YFP expression becomes down regulated as vessels form (N-Q). cxcr4a:YFP expression is maintained in large vessels and this expression persists late into adulthood after the coronary vasculature has formed (P and Q). cxcr4a:YFP (peudocolored blue), myl7:DsRed2-NLS (red) and fli1a:EGFP (green) in J, K, N-Q. Colorimetric display is used to show cxcr4a:YFP intensity (L). Single cxcr4a:YFP transgenic fish (M; YFP, yellow; PMT, grey). Scale bars, 50 µm. |
|
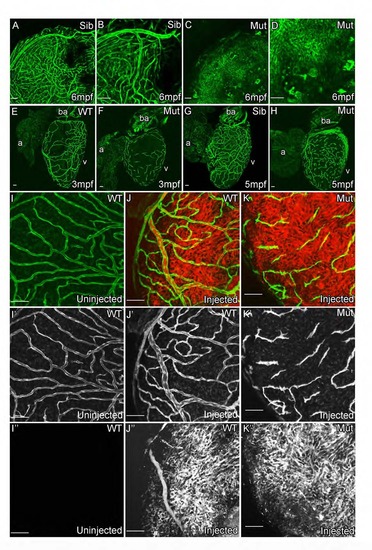
cxcr4a mutant zebrafish fail to develop functional coronary vasculature. (Related to Figure 5) kdrl:grcfp allows visualization of the endocardium, bright atrial vessels and weaker expressing non-arterial vessels in 6mpf sibling adult zebrafish (A and B). This network of vessels is not detectable in the cxcr4a mutant zebrafish; instead unorganized endothelial cells are detectable on the surface of the heart (C and D). These cells fail to form vessels and remain as isolated cells into adulthood (E-H). Angiographs suggest that there is no major supply of blood to the surface of the heart in the mutant other than the systemic flow [I-K; Green, fli1a:EGFP (I′-K′); Red, retro-orbitally injected Rhodamine-Dextran (I′′-K′′)]. Scale bars, 50 µm. |
|
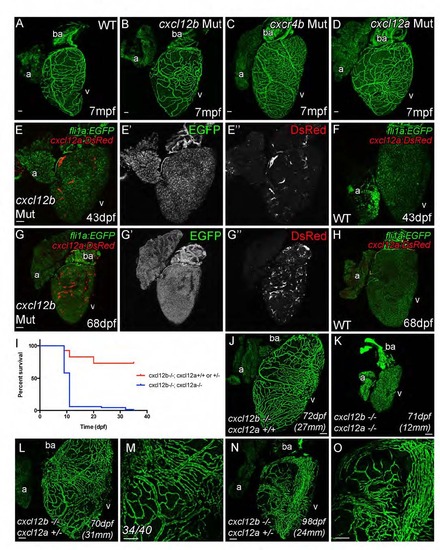
Coronary vasculature develops normally cxcr4b mutants, but cxcl12b and cxcl12a act redundantly. (Related to Figure 5) Hearts from zebrafish with genetic lesions in cxcl12b (B), cxcr4b (C) and cxcl12a (D) are phenotypically indistinguishable from those of non-mutant fli1a:EGFP transgenic zebrafish (WT, A). Cardiomyocyte expression of cxcl12a:DsRed is observed in cxcl12b mutant hearts, which could provide an alternative guidance source in the absence of Cxcl12b (46dpf, E and F; 68dpf, G and H). Cxcl12b and Cxcl12a appear to have general redundancy as loss of both genes prevents survival beyond one-month post fertilization under standard husbandry conditions (I). Double cxcl12a/b mutants maintained under optimized husbandry conditions can survive beyond one month (7 out of 136), but fail to develop coronary vasculature (K). The majority of zebrafish with a single copy of cxcl12a (and no cxcl12b) develop normal vasculature (L and M, 34/40), but some cxcl12a +/-; cxcl12b -/- do appear to have subtle abnormalities in vessel formation and positioning (N and O). Italicized age indicates zebrafish are raised using optimized husbandry, body length in parentheses. Scale bars, 50 µm. |
|
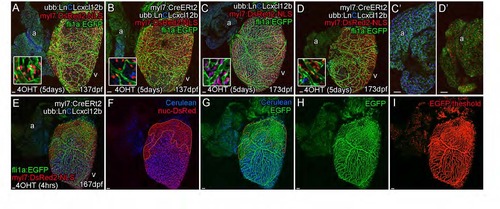
Cxcl12b directs coronary vessel formation. (Related to Figure 6) Uniform over-expression of cxcl12b resulted in a reduction in vessels and endothelial/vessel density in adult fish. However, this measurable reduction in endothelial cell density on the surface of the ventricle was not observed in older adult zebrafish over-expressing cxcl12b (137dpf A and B; 173dpf C and D). Ectopic expression of cxcl12b in atrial cardiomyocytes is not sufficient to direct vessel development on the surface of the atrium (C′ and D′, intensity levels adjusted to allow visibility of weaker atrial expression levels). Clonal overexpression of Cxcl12b in cardiomyocytes results in a significant increase in vessel density over the expressing region (E-I). Cxcl12b expression is indicated by the loss of H2B-Cerulean in myl7:DsRed2-NLS-positive cardiomyocytes (E-G, demarked by orange outline). Increase in density was measured using threshold level to define and estimate fli1a:EGFP-positive pixels that were part of the vascular network (H and I) and comparing this density between cxcl12b-expressing and non-expressing regions (demarcated by orange in this example, F and G) within an induced heart (paired t-test) and with siblings which lacked the myl7:CreERt2 transgene (non-paired t-test). Scale bars, 50 µm. |
Reprinted from Developmental Cell, 33, Harrison, M.R., Bussmann, J., Huang, Y., Zhao, L., Osorio, A., Burns, C.G., Burns, C.E., Sucov, H.M., Siekmann, A.F., Lien, C.L., Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish, 442-54, Copyright (2015) with permission from Elsevier. Full text @ Dev. Cell