- Title
-
Differentiation of the vertebrate retina is coordinated by an FGF signaling center
- Authors
- Martinez-Morales, J.R., Del Bene, F., Nica, G., Hammerschmidt, M., Bovolenta, P., and Wittbrodt, J.
- Source
- Full text @ Dev. Cell
|
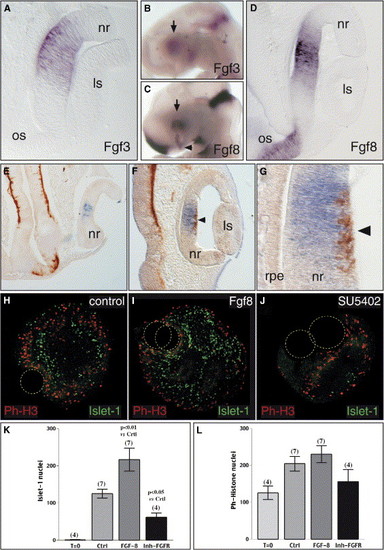
Fgfs Are Candidate Genes for Setting the Origin of RGC Differentiation in the Embryonic Chick Retina (A–D) During development both Fgf3 (stage 16, [A], and arrow in [B]) and Fgf8 (stage 17, [D], and arrow in [C]) are expressed in the central retina. (E–G) In double-staining experiments, Fgf8 expression (blue in [E], [F], and [G]) and β-tubulin (Tuj1) immunoreactivity (brown in [E], [F], and [G]) were examined shortly (stage15, [E]) before and (stage 18, [F] and [G]) after the onset of neuronal differentiation. Fgf8 expression (E) precedes and later (F and G) overlaps with the initial burst of neurogenesis (arrowheads in [F] and [G]). (H–J) The influence of Fgf signaling on RGC differentiation was analyzed quantitatively in vitro by coculturing retinal explants with heparin beads soaked in (H) PBS, (I) Fgf8, and (J) SU5402. (K and L) The number of islet-1-positive RGCs and phosphohistone-H3 (Ph-H3)-positive mitotic cells was determined by double immunohistochemistry. (L) Quantitative analysis from different (number indicated in brackets on each bar) experiments demonstrates that Fgf8 and SU5402 do not significantly modify the proliferation rate (Ph-H3-positive nuclei) in the explants. Note instead the dramatic effect on RGC differentiation (islet-1-positive nuclei; [K]). ls, lens; nr, neural retina; os, optic stalk; rpe, retinal pigmented epithelium. |
|
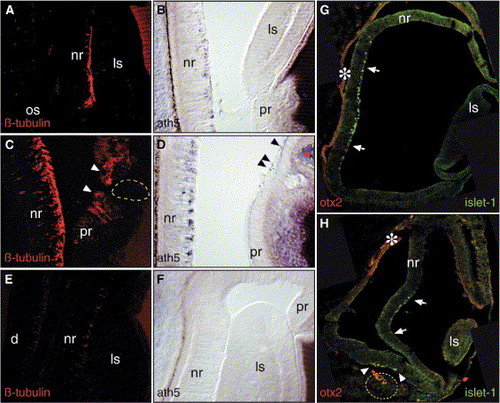
Local Exposure to Fgf8 Triggers New Foci of RGC Differentiation in the Embryonic Chick Eye (A–H) (A, B, and G) Control, (C, D, and H) FGF8, and (E and F) SU5402 bead-implanted embryos were sectioned and stained for (A, C, and E) β-tubulin, (B, D, and F) ath5, (G and H) islet-1, and Otx2 (red, labeled with an asterisk in [G] and [H]). Note that in the peripheral retina both β-tubulin and Cath5 are induced in proximity to the implanted Fgf8 bead (arrowheads in [C] and [D]). (E and F) SU5402-soaked beads inhibit neuronal differentiation. Inhibition occurs even at long range; the SU5402-soaked bead implanted is not visible within the photographed field. When beads are implanted into the periocular mesenchyme close to the RPE, Fgf8 induces an additional center of neurogenesis (arrowheads in [H]). d, diencephalon; pr, peripheral retinal. |
|
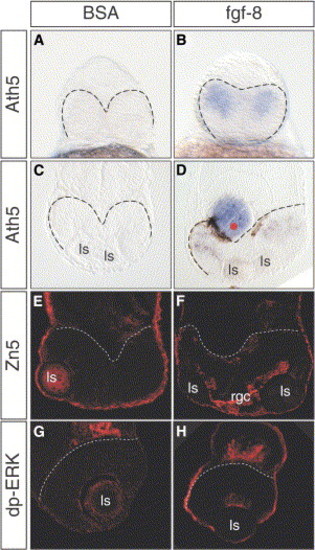
Fgf8 Rescues Retinal Differentiation in oep Mutants (A–H) (A and B) Frontal views and (C–H) coronal sections of oep embryos after (A–D) ath5 whole mount in situ staining, (E and F) Zn5, or (G and H) dp-ERK antibody staining at 28, 42, and 36 hpf, respectively. No ath5 expression is detected after implantations of BSA-soaked control beads ([A] and [C], 12 out of 12 embryos). In contrast, ath5 expression was rescued upon implantation of Fgf8-soaked beads ([B] and [D], 9 out of 11 embryos). Similarly, the RGC marker Zn5 was detected in embryos implanted with (F) Fgf8 beads, but not in those that received (E) control beads. (G and H) Dp-ERK staining was also absent in control embryos and was rescued by Fgf8 bead implantation. Dashed lines demarcate the posterior edge of the neuroretina. Rgc, retinal ganglion cells. |
|
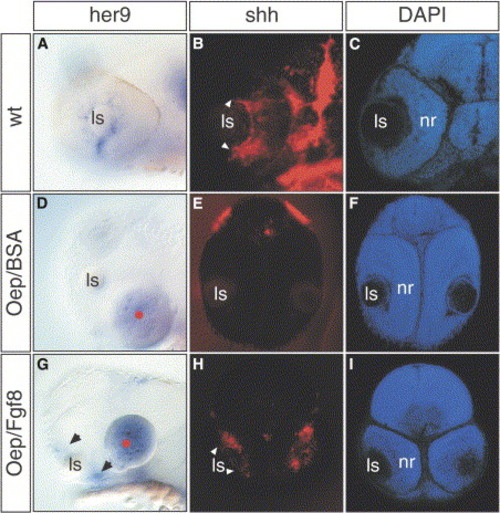
Fgf8 Rescues her9 and shh Expression in the oep Retina (A–I) Lateral views of (A) wild-type and (D and G) oep mutant embryos at 32 hpf. (A) her9 is normally expressed in the neuronal progenitors located in the most peripheral part of the ciliary marginal zone, close to the lens. Its expression is absent in oep mutants implanted with control beads (d, 0 out of 9), but is rescued following Fgf8 bead implantation ([G], 9 out of 10). Frontal sections of 42 hpf (B and C) wild-type and (E, F, H, and I) oep mutants after (B, E, and H) shh whole-mount in situ hybridization and (C, F, and I) DAPI counterstaining. shh is expressed in the most central part of the neural retina in a subpopulation of cells in the retinal ganglion cell and inner nuclear layers (arrowheads in [B]). This expression is absent in (E) oep control implanted embryos, but is rescued when (H) Fgf8 beads are implanted. (B and H) White arrowheads indicate the front of shh expression. (G) Black arrowheads indicate the rescue of her9 expression in the peripheral retina of oep embryos. |
|
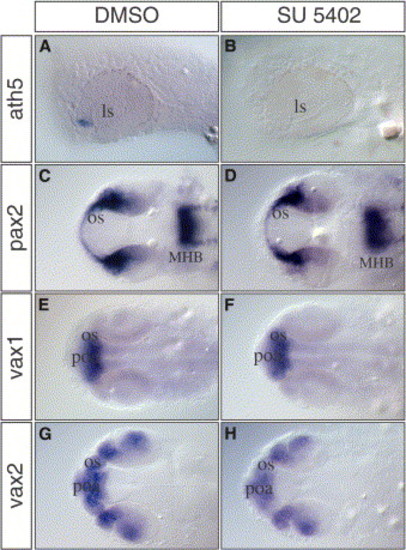
Blocking Fgf Signaling Prevents the Initiation of Retina Differentiation (A–H) (A and B) Lateral and (C–H) dorsal views of zebrafish embryos treated for 5 hours (23–28 hpf) with the (B, D, F, and H) Fgf signaling inhibitor SU5402 or (A, C, E, and G) mock solution. (A and B) In contrast to the control embryos, ath5 expression is absent in SU5402-treated embryos. Other genes expressed in the optic stalk (pax2, vax1, and vax2) were not affected by SU5402 treatment (compare [D], [F], and [H] to [C], [E], and [G], respectively). MHB, midbrain-hindbrain boundary; poa, pre-optic area. EXPRESSION / LABELING:
|
|
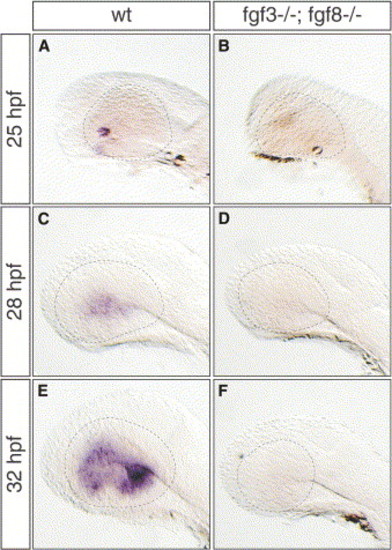
Fgf3 and Fgf8 Cooperate in Initiating Retina Differentiation (A–F) Lateral views of (B, D, and F) Fgf3 and Fgf8 double mutant embryos and (A, C, and E) wild-type siblings at 25 hpf, 28 hpf, and 32 hpf hybridized with an ath5-specific probe. In contrast to wild-type, ath5 transcripts are absent in mutant embryos (compare [B], [D], and [F] to [A], [C], and [E], respectively). Dashed lines demarcate the outlining of the eye. |
|
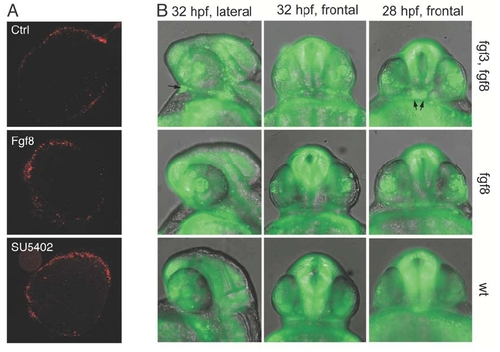
(A) Cell death was examined in control explants and explants exposed to FGF8 or SU5402 by propidium iodide staining. Note that the amount of cell death is minimal and basically limited to the edges in all explants under the experimental conditions employed. PHENOTYPE:
|
|
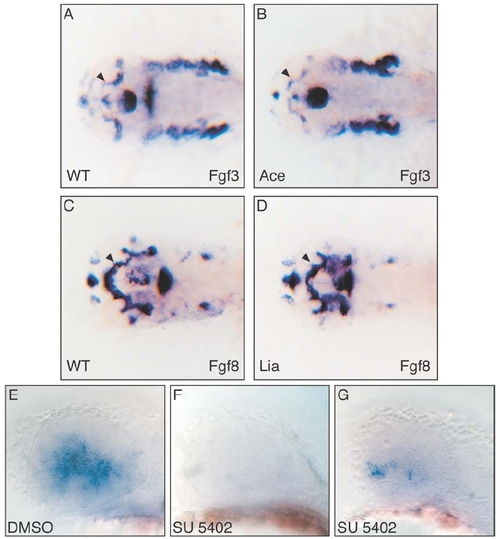
Expression of Fgf3 and Fgf8 in zebrafish Fgf mutant embryos. The expression of Fgf3 and Fgf8 was analyzed in Ace/Fgf8 and Lia/Fgf3 mutant embryos, respectively. No difference was observed in the expression of these genes in the optic stalk region, indicated by arrowheads (compare A with B, and C with D). This excludes any feedback cross regulation or genetic hierarchy between these two genes in this domain. Inhibition of Fgf signalling prevents Ath5 expression at 32 hpf. Drug treatment with the Fgf inhibitor SU5402 abolishes or strongly reduces Ath5 expression (compare E with F and G). Embryos where treated from 23 to 32 hpf. The mock DMSO treated embryos showed always an extended Ath5 expression (E, 12 out of 12) while the SU5402 treatment either prevents Ath5 expression (F, 5 out of 11) or results in a strongly reduced expression (G, 6 out of 11).EXPRESSION / LABELING:
|
|
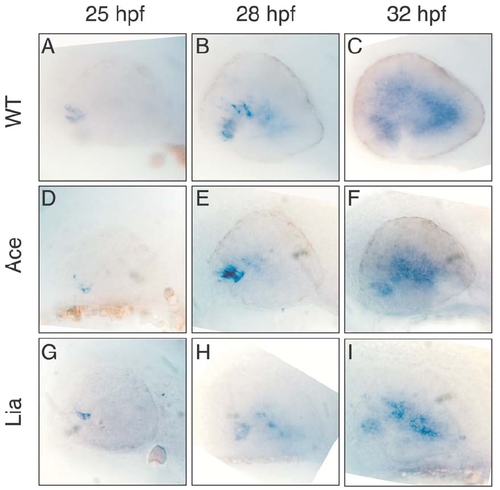
Expression of Ath5 in zebrafish wt and Fgf mutant embryos. The temporal and spatial expression of Ath5 was examined in wild type (A,B,C), Ace/Fgf8 (D,E,F) and Lia/Fgf3 (G,H,I) mutant embryos. No significant difference was observed at 25 hpf and 28 hpf (A, D, G and B, E, H, respectively). However by 32 hpf a slight decrease in Ath5 expression was observed in Ace and Lia mutants (compare C with F and I). This could be due to a secondary developmental defect of these embryos or alternatively it would support a partially non-redundant role of Fgf8 and Fgf3 for the correct progression of Ath5 expression and retinal ganglion cell differentiation. |
|
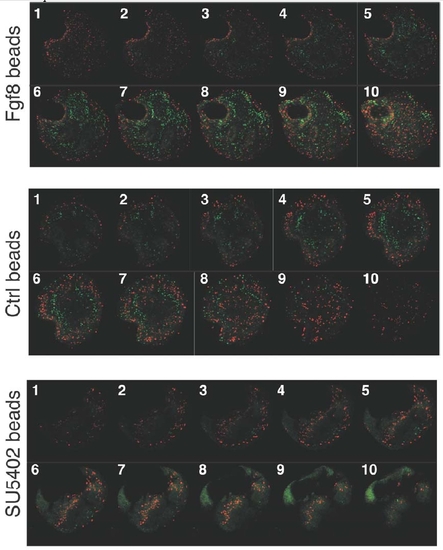
A complete sequence of 10 consecutive confocal optical sections is shown for explants (Figure 1) exposed to beads soaked in Fgf8, PBS (control), and SU5402. Note the clear differences in the ratio between the numbers of differentiated cells (islet-1, green nuclei) versus proliferating cells (phospho-Histone 3, red nuclei) in those explants. |
Reprinted from Developmental Cell, 8(4), Martinez-Morales, J.R., Del Bene, F., Nica, G., Hammerschmidt, M., Bovolenta, P., and Wittbrodt, J., Differentiation of the vertebrate retina is coordinated by an FGF signaling center, 565-574, Copyright (2005) with permission from Elsevier. Full text @ Dev. Cell