- Title
-
A cell-and-plasma numerical model reveals hemodynamic stress and flow adaptation in zebrafish microvessels after morphological alteration
- Authors
- Maung Ye, S.S., Phng, L.K.
- Source
- Full text @ PLoS Comput. Biol.
|
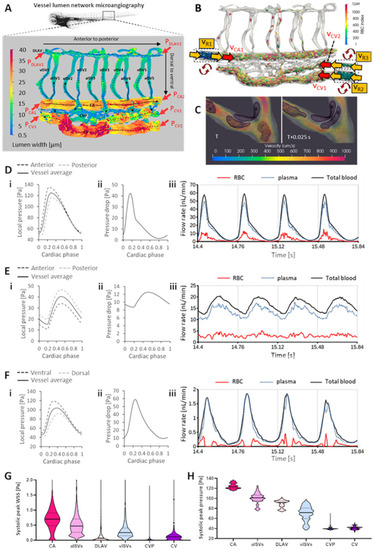
Development of the cell-and-plasma 3-D computational fluid dynamics (CFD) model |
|
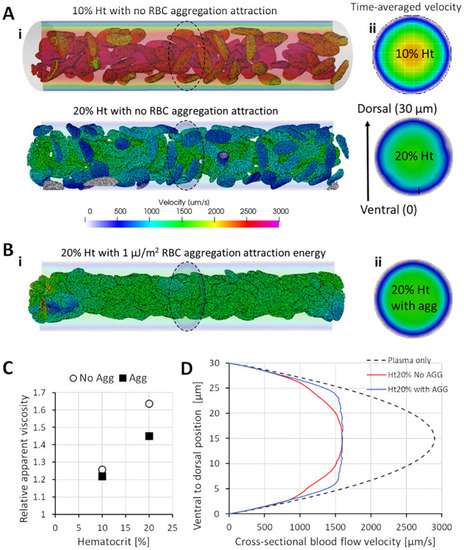
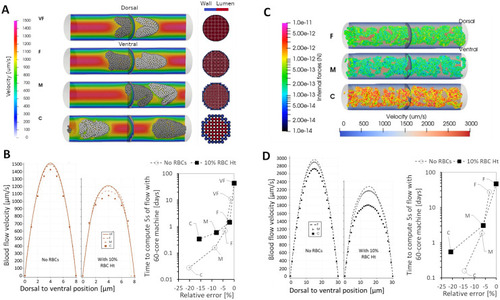
Effects of RBC hematocrit and aggregation on viscosity and blood flow velocity. |
|
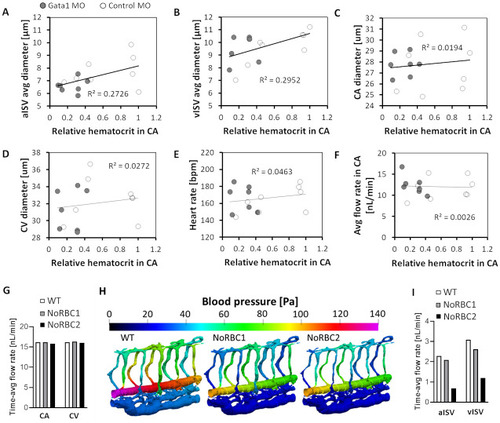
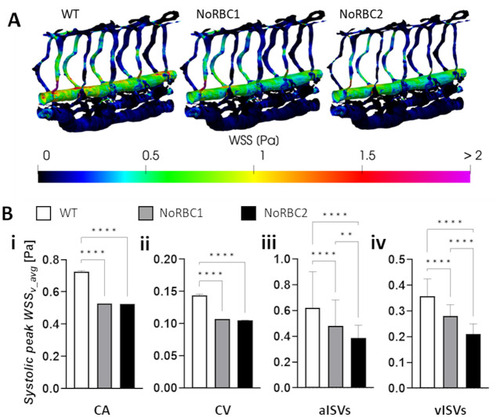
Systemic alteration in vessel morphology and blood flow in zebrafish with reduced RBC hematocrit. |
|
Decrease in ISV diameter exacerbates the decrease in WSS caused by hematocrit reduction. |
|
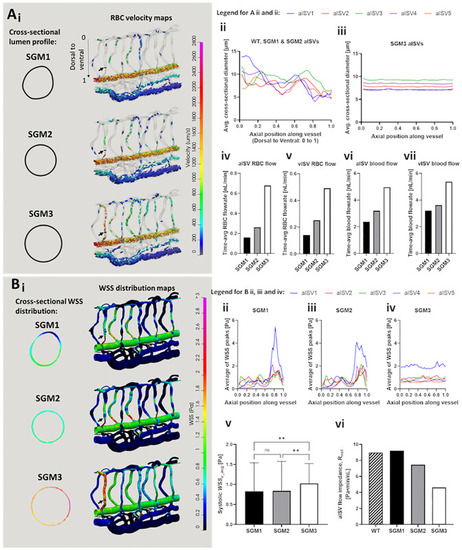
Simulation predictions of flow and WSS distribution in networks with ISVs of varying diameters and cross-sectional shapes. (see also |
|
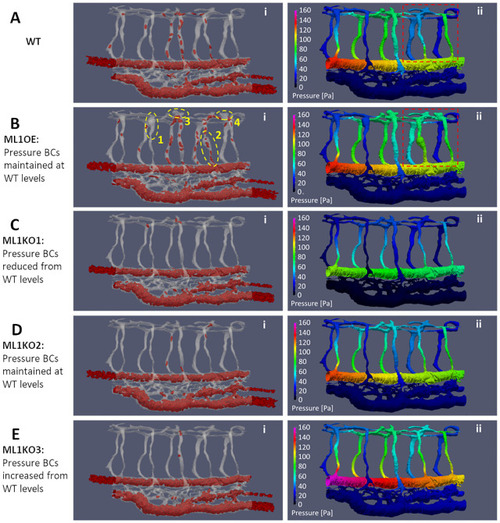
Hemodynamics adaptation scenarios for Marcksl1 OE and Marcksl1 KO. |
|
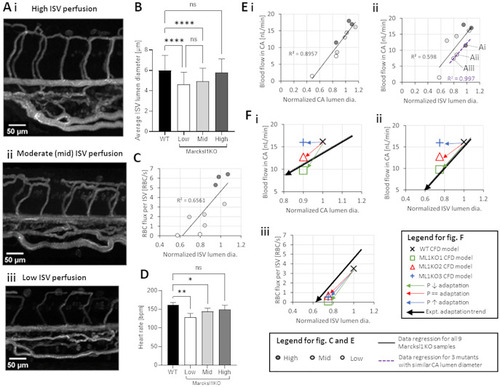
Characterization of RBC flow in Marcksl1 KO zebrafish with reduced vessel diameters. |
|
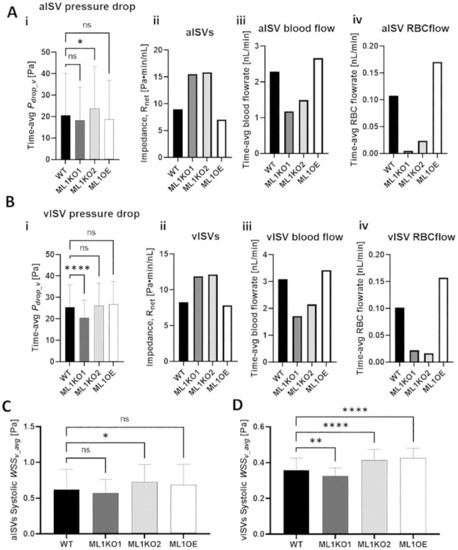
Comparison of pressure and WSS distributions in ISVs amongst WT and Marcksl1 network models. |
|
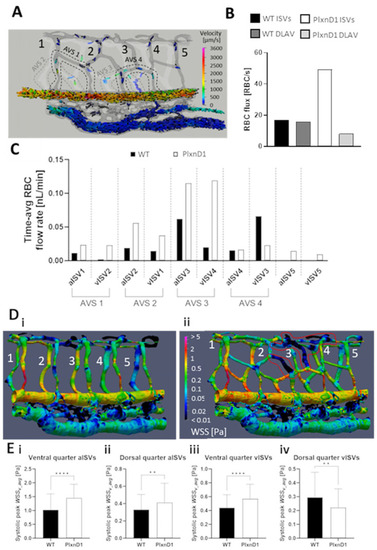
Hemodynamic alterations arising from network mispatterning in PlxnD1 model (see |
|
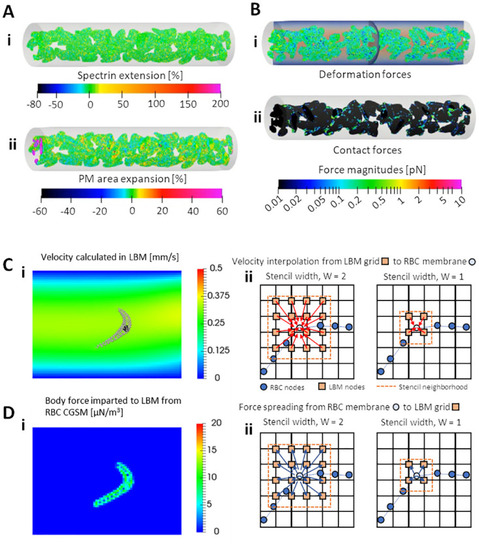
The fundamental components and physics of the cell-and-plasma phase CFD model. |
|
Grid independence testing for numerical accuracy and precision in the simulations. |