- Title
-
Pou2, a class V POU-type transcription factor in zebrafish, regulates dorsoventral patterning and convergent extension movement at different blastula stages
- Authors
- Khan, A., Nakamoto, A., Okamoto, S., Tai, M., Nakayama, Y., Kobayashi, K., Kawamura, A., Takeda, H., and Yamasu, K.
- Source
- Full text @ Mech. Dev.
|
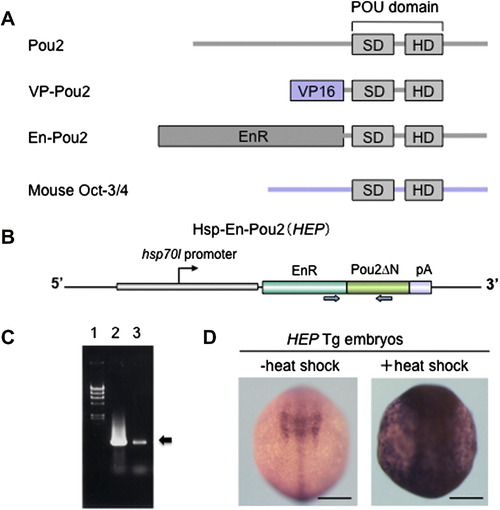
The strategy of the overexpression of pou2 and its related genes. (A) Structures of the proteins encoded by pou2, vp-pou2, en-pou2, and mouse Oct-3/4. SD, POU-specific domain; HD, POU homeodomain. (B) The structure of the Hsp-En-Pou2 gene (HEP). The PCR primers used to detect the HEP transgene by genotyping of embryos are shown by a pair of blue arrows. (C) Identification of the HEP-containing transgenic (Tg) fish by detecting the transgene sequence using PCR in offspring embryos followed by agarose gel electrophoresis (arrow). 1, Phage lambda DNA digested by HindIII, used as size markers; 2, PCR product obtained using the HEP construct as a template; 3, PCR product obtained by genotyping of offspring embryos from HEP Tg fish. (D) HEP Tg embryos were exposed to heat shock for 1 h (9 hpf) and examined 1 h later (bud stage) for the expression of pou2 by whole-mount in situ hybridization. When embryos were not exposed to high temperature (–heat shock), the endogenous pou2 expression alone was detected, whereas heat treatment induced pou2 expression throughout the embryo (+heat shock). Scale bar, 200 μm. |
|
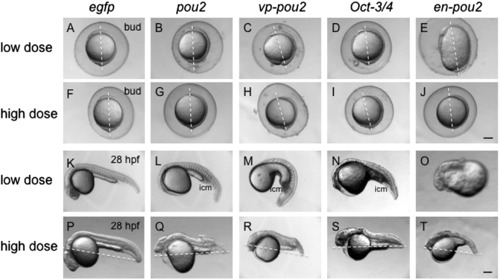
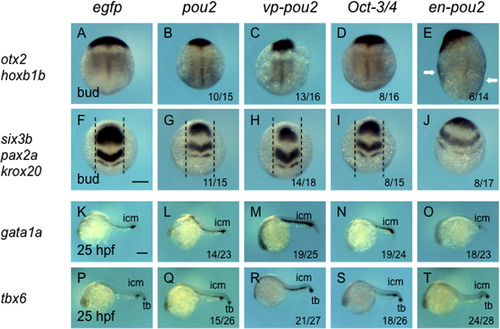
Morphological effects of the overexpression of pou2 and its related genes by mRNA injection. mRNA at a low dose (30 pg/embryo, A–E, K–O) and a high dose (90 pg/embryo, F–J, P–T) for egfp (A, F, K, P), pou2 (B, G, L, Q), vp-pou2 (C, H, M, R), mouse Oct-3/4 (D, I, N, S), and en-pou2 (E, J, O, T) was injected into embryos, which were observed at the bud stage (A–J) and 28 hpf (K–T). Lateral views with anterior to the top and dorsal to the right (A–J) or with anterior to the left and dorsal to the top (K–T). (A–J) Dashed lines show animal–vegetal axes, emphasizing incomplete anterior migration of the neural plate (F–J), or connect the tail tips and the centers of the yolk balls, pointing to extension defects of the body axes, in embryos overexpressing a high dose of all four pou2-related genes. icm, intermediate cell mass. Scale bar, 200 μm. |
|
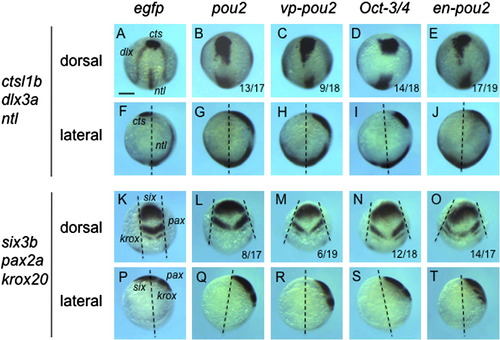
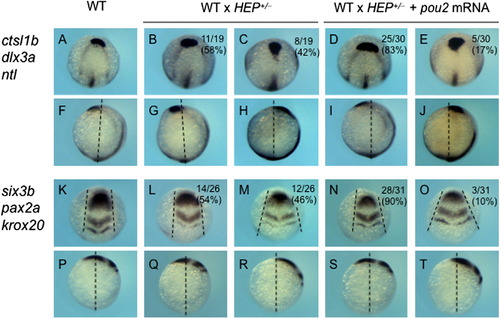
Expression of axial and neural genes in embryos injected with high-dose mRNA for pou2-related genes. The expression of axial and neural genes, shown on the left, was examined at the bud stage in embryos injected with high-dose mRNA for egfp (A, F, K, P), pou2 (B, G, L, Q), vp-pou2 (C, H, M, R), mouse Oct-3/4 (D, I, N, S), and en-pou2 (E, J, O, T). The numbers of embryos with abnormal expression patterns and total numbers of embryos examined are shown at the bottom of the respective panels. (F–J, P–T) Dashed lines represent the animal-vegetal axes, showing that pou2-related genes affected anterior extension of the embryo. (K–O) Pairs of dashed lines show lateral edges of the neural plate, revealing that convergence of the neural plate is affected by overexpression of pou2-related genes. Scale bar, 200 μm. |
|
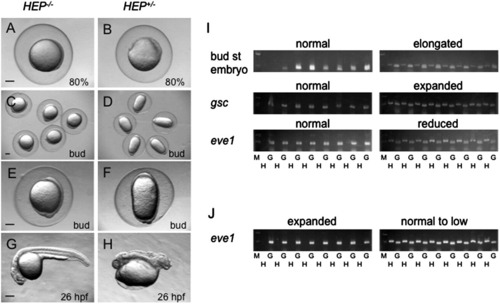
Effects of HEP induction on early aspects of zebrafish development. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to high temperature for 1 h and then allowed to develop at a lower temperature to appropriate stages. (A, B) Induction of HEP at the high/oblong stage led to epiboly arrest and thickening of the blastoderm in about half of the embryos (B) when the remaining embryos were normal and reached 80% epiboly (A). (C–H) Induction of HEP at the sphere stage led to elongation of embryos along the animal–vegetal axis at the bud stage in about half of the embryos (D, F), while the remaining embryos were normal (C, E). By 26 hpf, many of elongated embryos had died and live embryos were severely disrupted in their posterior region (compare G and H). Scale bar, 200 µm. (I, J) Genotyping of embryos exposed to heat shock at the sphere stage. G, genomic sequences amplified for positive control; H, HEP-derived sequences showing that the embryos harbored the HEP gene; M, size markers (500 and 200 bp). (I) Embryos that were normal or abnormal in terms of morphology and DV marker expression were subjected to genotyping. The data demonstrated that normal embryos were negative but abnormal embryos were positive for HEP. (J) Offspring embryos from crosses of HEP+/- Tg and wild-type fish were injected with pou2 mRNA and then given heat shock at the sphere stage. Genotyping showed that the embryos with expanded eve1 expression were negative (+++; Table 2), and the embryos with slightly reduced expression (+) were positive for HEP. |
|
Dorsalization of embryos by HEP induction at the sphere stage. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to heat shock at the sphere stage, allowed to develop to the bud stage at a lower temperature, and then examined for the expression of dorsal markers (right two columns). (A–D) Frontal views with dorsal to the top. (E–L, M–P) Dorsoanterior views with anterior to the top. (I2–L2, M2–P2) Dorsoposterior views with anterior to the top. Embryos in the third column showed normal patterns (Sibling-HS), whereas those in the rightmost column showed abnormal expression (HEP-HS). The number of embryos with abnormal expression and total embryo number are shown at the bottom of the respective panels in the HEP-HS column. For the control, gene expression was examined in parallel in wild-type embryos that were untreated (left column, Wild) or treated with heat shock (second column, Wild-HS). Scale bar, 200 μm. |
|
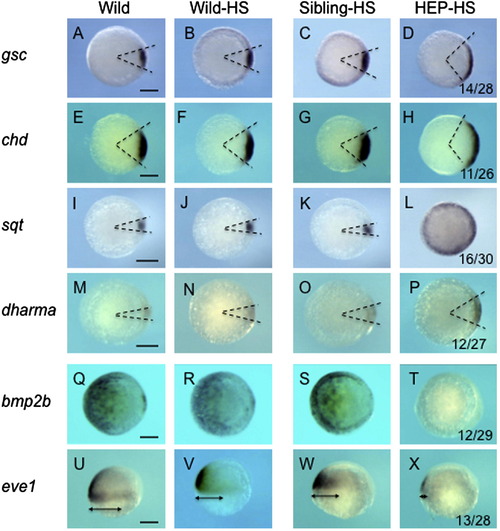
Effects of HEP induction at the sphere stage on the expression of dorsoventral patterning genes. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to heat shock at the sphere stage for 1 h, allowed to develop at a lower temperature to 50% epiboly, and then examined for the expression of DV patterning genes (right two columns). Embryos in the third column showed normal patterns (Sibling-HS), whereas those in the rightmost column showed expanded (D, H, L, P) or reduced (T, X) expression (HEP-HS). The numbers of embryos with abnormal expression and total numbers of embryos examined are shown at the bottom of the respective panels in the HEP-HS column. For the control, gene expression was examined in parallel in wild-type embryos that were untreated (left column, Wild) or treated with heat shocks (second column, Wild-HS). Scale bar, 200 μm. |
|
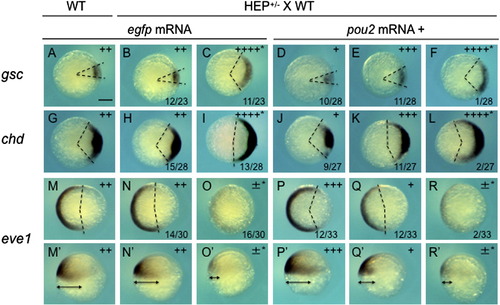
Rescue of the dorsalizing effects of HEP induction at the sphere stage by pou2 overexpression. Embryos from crosses of wild-type (WT) and HEP+/- transgenic (Tg) fish were injected with egfp (second and third column) or pou2 mRNA (low dose, 30 pg/embryo; fourth to sixth column), exposed to heat shock at the sphere stage, and allowed to develop at lower temperature to the shield stage, at which time they were examined for the expression of DV patterning genes. For the control, wild-type embryos (WT) were stained likewise (leftmost column). Embryos expressing egfp showed either normal (second column) or abnormal expression of DV patterning genes (asterisks, third column). The abnormal expression can be ascribed to HEP induction. When pou2 mRNA was injected in advance, the ratios of embryos showing HEP-dependent effects (asterisks) were greatly decreased (rightmost column). ±, +. ++. +++, ++++, see the footnotes for Table 2. |
|
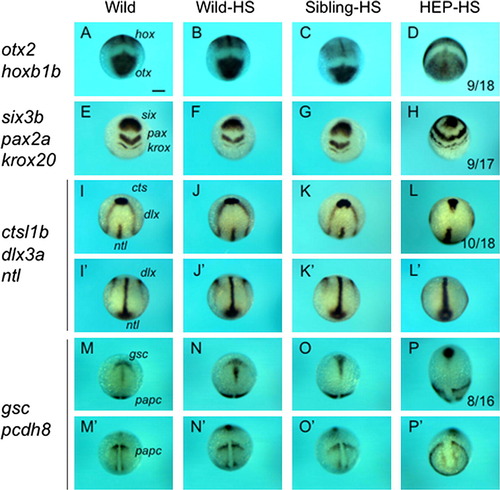
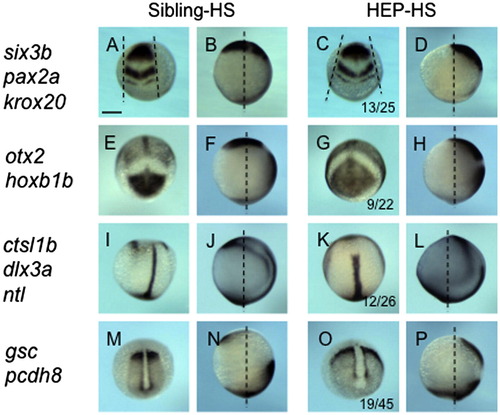
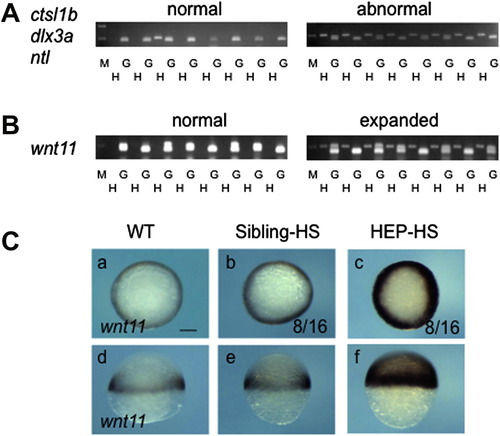
Effects of HEP induction at 30% epiboly on CE movement. Embryos from crosses of wild-type and HEP+/- fish were exposed to heat shock at 30% epiboly, allowed to develop to the bud stage at a lower temperature, and then examined for the expression of regional markers. Embryos in the left two columns showed normal patterns (Sibling-HS), whereas those in the right two columns showed abnormal patterns (HEP-HS), typical of embryos with affected CE movement. (A, C, I, K, M, O) Dorsal views with anterior to the top. (E, G) Frontal views with dorsal to the top. (Second and fourth columns) Lateral views with anterior to the top and dorsal to the right. The number of embryos with abnormal expression and the total numbers of embryos are shown in the third column. Scale bar, 200 μm. |
|
HEP induction at 30% epiboly upregulates wnt11 expression at the blastoderm margin. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to heat shock at 30% epiboly and later examined for gene expression. (A, B) Embryos that were normal or abnormal in terms of gene expression at the bud stage (A) or 50% epiboly (B) were subjected to genotyping. The data demonstrated that normal embryos were negative and abnormal embryos were positive for HEP. G, genomic sequences amplified for the positive control; H, HEP-derived sequences showing that the embryos were transgenic; M, size markers (500 and 200 bp). (C) Embryos subjected to heat shock were examined for wnt11 expression (right two columns). About half of the embryos showed extensive upregulation (HEP-HS). (a–c) Animal views. (d–f) Lateral views. Scale bar, 200 μm. |
|
pou2 overexpression rescues the effects of HEP induction at 30% epiboly. Embryos from crosses of wild-type (WT) and HEP+/- transgenic (Tg) fish were injected with egfp (second and third column) or pou2 mRNA (low dose, 30 pg/embryo; fourth and fifth column), exposed to heat shock at 30% epiboly, and allowed to develop until the bud stage at a lower temperature, at which time they were examined for marker expression. For the control, wild-type embryos (WT) were similarly stained (leftmost column). About half of the offspring embryos injected with egfp mRNA showed CE defects (third column). When pou2 mRNA was injected in advance, the ratio of embryos showing HEP-dependent effects was greatly decreased (rightmost column). |
|
Arrest of the epiboly in embryos overexpressing en-pou2. Epiboly was arrested in embryos injected with a low dose (30 pg/embryo) and high dose (90 pg/embryo) of en-pou2 mRNA. Scale bar, 200 μm. |
|
Expression of dorsoventral marker genes in embryos injected with mRNA for pou2-related genes. The expression of dorsoventral marker genes, shown on the left, was examined at the bud stage (A–J) or at 25 hpf (K–T) in embryos injected with mRNA for egfp (A, F, K, P), pou2 (B, G, L, Q), vp-pou2 (C, H, M, R), mouse Oct-3/4 (D, I, N, S), and en-pou2 (E, J, O, T). The numbers of embryos with abnormal expression patterns and total numbers of embryos examined are shown at the bottom of the respective panels. (A–J) The expression of neural markers in the dorsal neural plate became narrower in embryos overexpressing pou2, vp-pou2, and Oct-3/4, whereas it expanded ventrally in en-pou2-overexpressing embryos. In embryos overexpressing en-pou2 (E), hoxb1b expression was expanded ventrally, which can be seen in the flank region as marked by white arrows. (F-I) The intervals between parallel dashed lines are the same. (K–T) The expression of gata1a and tbx6 in the ICM was expanded by pou2, vp-pou2, and Oct-3/4, but downregulated by en-pou2. tbx6 expression in the tail bud was little affected. icm, intermediate cell mass; tb, tail bud. Scale bar, 200 μm. |
|
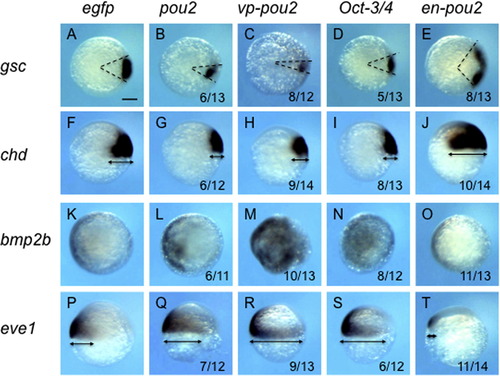
Expression of dorsoventral patterning genes in early embryos injected with low-dose mRNA for pou2-related genes. The expression of dorsoventral patterning genes, shown on the left, was examined at the shield stage in embryos injected with low-dose (30 pg/embryo) mRNA for egfp (A, F, K, P), pou2 (B, G, L, Q), vp-pou2 (C, H, M, R), mouse Oct-3/4 (D, I, N, S), and en-pou2 (E, J, O, T). Animal views with dorsal to the right (A–E, K–O) or lateral views with dorsal to the right and anterior to the top (F–J, P–T). The numbers of embryos with abnormal expression patterns and total numbers of embryos examined are shown at the bottom of the respective panels. The angles between the two dashed lines show the sizes of the gsc domain (A–E), and the arrows show the DV extent of the expression of chd (F–J) and eve1 (P–T). The expression of dorsal genes was reduced and ventral gene expression was expanded by pou2, vp-pou2, and mouse Oct-3/4. In contrast, en-pou2 overexpression gave rise to reverse effects. Scale bar, 200 μm. |
|
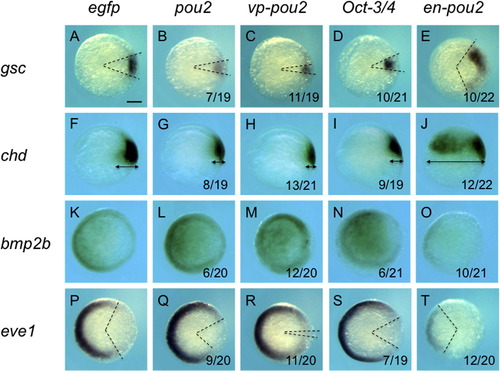
Expression of dorsoventral patterning genes in early embryos injected with high-dose mRNA for pou2-related genes. The expression of dorsoventral patterning genes, shown on the left, was examined at 50% epiboly-shield stage in embryos injected with high-dose mRNA (90 pg/embryo) for egfp (A, F, K, P), pou2 (B, G, L, Q), vp-pou2 (C, H, M, R), mouse Oct-3/4 (D, I, N, S), and en-pou2 (E, J, O, T). Animal views with dorsal to the right (A–E, K–T) or lateral views with dorsal to the right and anterior to the top (F–J). The numbers of embryos with abnormal expression patterns and total numbers of embryos examined are shown at the bottom of the respective panels. The angles between the two dashed lines show the sizes of the gsc (A–E) and eve1 domains (P-T), respectively, and the arrows show the extent of chd expression (F–J). The effects of high-dose mRNA for pou2-related genes were similar to those of low-dose mRNA of the same genes; the expression of dorsal genes was reduced and ventral gene expression was expanded by pou2, vp-pou2, and mouse Oct-3/4, whereas en-pou2 overexpression gave rise to reverse effects. Scale bar, 200 μm. |
|
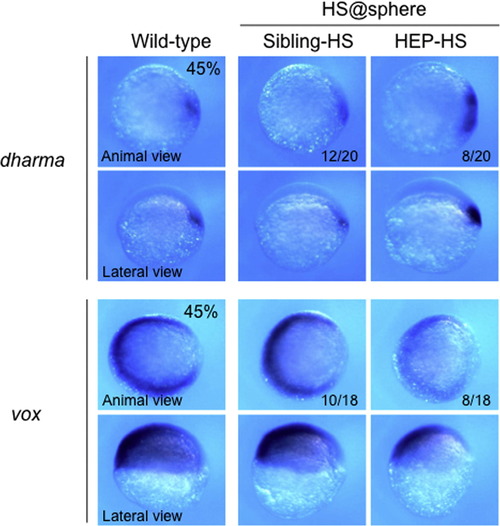
Expression of dharma and vox immediately after HEP induction at the sphere stage. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to heat shock at the sphere stage, allowed to develop to 45% epiboly at a lower temperature, and then examined for the expression of the genes specified on the left. The numbers of embryos with normal (second column, Sibling-HS) or abnormal (right column, HEP-HS) expression together with the total numbers of embryos examined are shown at the bottom of the respective panels. For the control, marker expression is shown for wild-type embryos (left column). The numbers of embryos with the expression patterns shown in the corresponding panels and total numbers of embryos examined are shown at the bottom of the respective panels. dharma expression was expanded, whereas vox was downregulated, immediately after HEP induction at the sphere stage. |
|
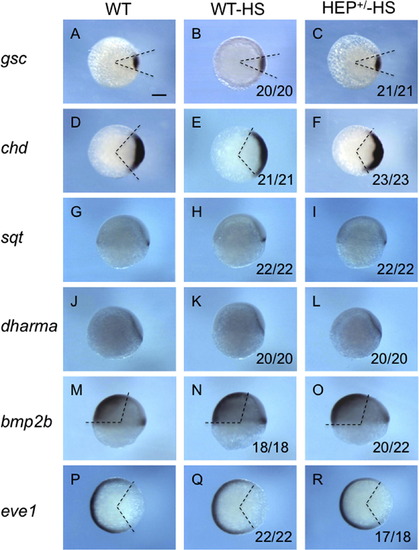
Dorsoventral patterning is not affected by HEP induction at 30% epiboly. Embryos from crosses of wild-type and HEP+/- transgenic (Tg) fish were exposed to heat shock at 30% epiboly, allowed to develop to the shield stage at a lower temperature, and then examined for the expression of dorsoventral marker genes (HEP+/-HS, right column). For the control, marker expression is shown for wild-type embryos (WT, left column) and for wild-type embryos exposed to heat treatment (WT-HS, middle column). (A-F, P-R) Animal views with dorsal to the right. (G-O) Lateral views with dorsal to the right and anterior to the top. The angles between the two dashed lines show the sizes of dorsoventral marker expression. The numbers of embryos with normal expression and total embryo numbers are shown at the bottom of the respective panels in the right two columns. Essentially all HEP-treated embryos showed normal marker expression, showing that HEP induction at 30% epiboly does not affect dorsoventral patterning. Scale bar, 200 μm. |
Reprinted from Mechanisms of Development, 129(9-12), Khan, A., Nakamoto, A., Okamoto, S., Tai, M., Nakayama, Y., Kobayashi, K., Kawamura, A., Takeda, H., and Yamasu, K., Pou2, a class V POU-type transcription factor in zebrafish, regulates dorsoventral patterning and convergent extension movement at different blastula stages, 219-235, Copyright (2012) with permission from Elsevier. Full text @ Mech. Dev.