- Title
-
Genetic developmental timing revealed by inter-species transplantations in fish
- Authors
- Fuhrmann, J.F., Buono, L., Adelmann, L., Morales, J.R.M., Centanin, L.
- Source
- Full text @ Development
|
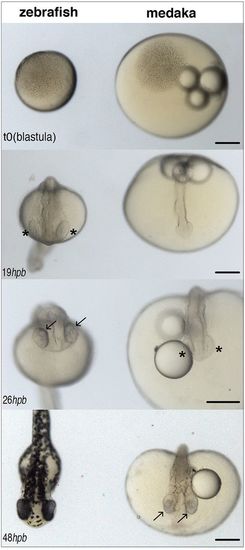
Different developmental timing for zebrafish and medaka. Images of zebrafish (left) and medaka (right) embryos synchronised at the blastula stage (top, 512 cells, t=0) and at different stages of embryonic development (hours post blastula, hpb). Eye cups (asterisks) are evident in zebrafish by 19 hpb and retinal pigmentation (and neurogenesis) (arrows) by 26 hpb; in medaka, retinal pigmentation does not start before 48 hpb. Scale bars: 200 µm. |
|
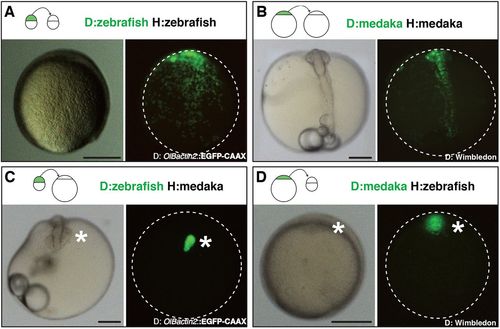
Intra- and inter-species transplantation of blastocysts in zebrafish and medaka. (A-D) Transmitted (left) and fluorescent (right) images of a non-labelled host embryo that was transplanted at a blastula stage with isochronic EGFP fluorescent cells. Images were taken after completion of initial morphogenesis (90% epiboly stage for zebrafish and early neurula stage for medaka), and transplantation schemes are displayed at the top of each panel. Animal side is up for zebrafish hosts (A,D) and anterior side is up for medaka hosts (B,C). Dispersed green dots in A,B are donor cells intermingled with host cells (n>20 transplantation events for each species, n>10 embryos per transplantation event; a transplantation event is a transplantation experiment performed on a given day with a specific donor-host combination that leads to one or more chimeras of the described phenotype). EGFP+ clusters in C,D (asterisks) contain donor cells that did not mix with host cells. Representative images were chosen out of n=10 zebraka and n=12 medrafish. Clusters of the donor cells were found at later developmental stages in n=17 transplantation experiments, n>100 chimeras for zebraka; n=33 transplantation experiments, n>100 chimeras for medrafish (see Tables S1 and S2). Scale bars: 200 µm. D, donor; H, host. |
|
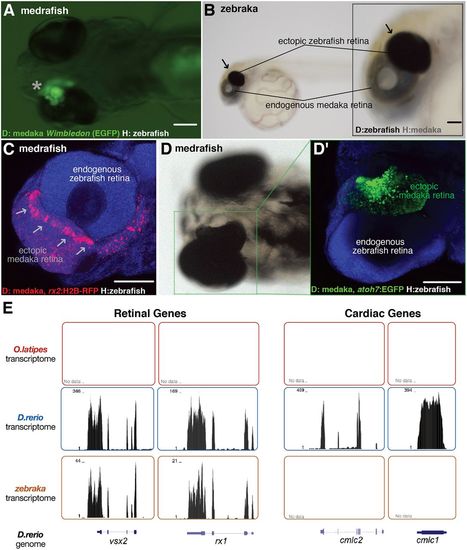
Transplanted EGFP+ cluster develops into an ectopic retina both in zebraka and medrafish. Images of medrafish (A,C,D) and zebraka (B). The EGFP+ cluster (asterisk, ventral view of a hatch embryo; A) and the pigmented cluster (arrows, lateral view of a hatched embryo; B) develop into an ectopic retina (n=45 transplantation events). Confocal images show expression markers of retinal progenitors (arrows in C; rx2:H2B-RFP donors in medrafish at 4 dpf) and retinal neurogenesis (D; atoh7:EGFP donors in medrafish at 5 dpf) (C,D′, DAPI in blue) (n=17 transplantation events). (E) Transcriptomes of medaka (top, n=2), zebrafish (middle, n=2) and zebraka (bottom, n=3) plotted along the zebrafish genome. The zebrafish cells in medrafish display retinal identity (vsx2 and rx1, left two panels) and no cardiac gene markers (cmlc1 and cmlc2, right panels). Scale bars: 100 µm. D, donor; H, host. |
|
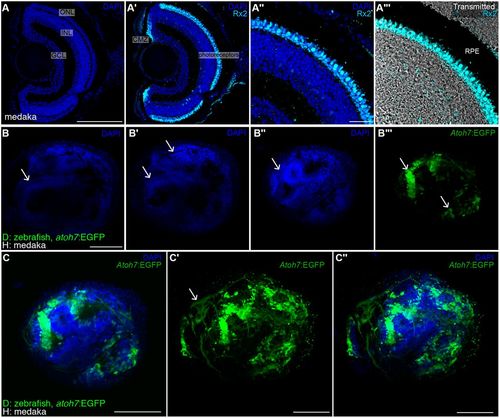
Topological organisation of retinal ganglion cells in ectopic retina. (A-A‴) DAPI and immunostaining using an anti-Rx2 antibody on a medaka retina cryosection. Retinal ganglion cells localise to the ganglion cell layer (GCL) and Rx2+ photoreceptors (cyan staining in A′-A‴) are found in the outer nuclear layer (ONL), adjacent to the retinal pigmented epithelium (black region in the transmitted channel, A‴). (B-C″) DAPI staining of whole-mount retinae in zebrakas shows conspicuous layering (arrows in B,B′) and clusters of retinal ganglion cells (arrows in B″,B‴) labelled in green using a Tg(atoh7:EGFP) zebrafish donor. Single plane (C) and stack (C′,C″) showing RGCs and their axons (arrow in C′) in an ectopic zebraka retina (n=6 retinae in six chimeras). Scale bars: 100 µm in A,A′,B-C″; 20 µm in A″,A‴. GCL, ganglion cell layer; INL, inner nuclear cell layer; ONL, outer nuclear cell layer; CMZ, ciliary marginal zone; RPE, retinal pigmented epithelium; D, donor; H, host. |
|
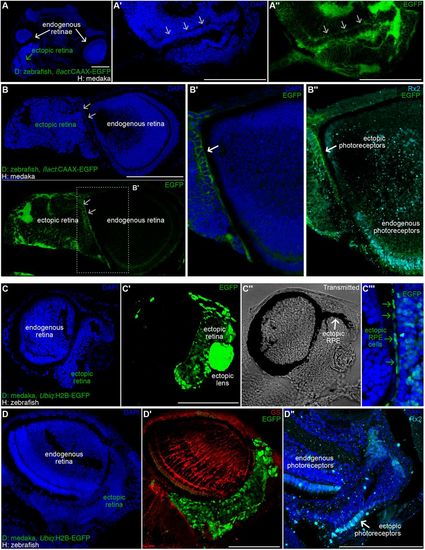
Partial layering in the ectopic retinae of zebraka and medrafish. (A-B″) DAPI staining on cryosections of zebrakas using Tg(ßact:CAAX-EGFP) zebrafish as donors. (A) Cryosection of a transverse plane in a zebraka (dorsal is upwards, anterior is to the front) showing an ectopic retina (green arrow) ventrally adjacent to an endogenous retina (white arrow). Layering is evident in the ectopic retinae both by nuclear morphology (DAPI staining, arrows in A′ and B, top) and by membrane accumulation (CAAX-EGFP, arrows in A″, B, bottom, and B′) (n=6 ectopic retinae in 6 zebrakas). Immunostaining using anti-Rx2 Ab reveal photoreceptor identity of cells organised in layers (B″, white arrow) (n=3 zebrakas). (C-D″) DAPI staining (C,D) on cryosections of medrafish using Tg(Ubiq:H2B-EGFP) medaka as donors. (C′,D′) EGFP signal allows detecting ectopic cells. Transmitted channel (C″) analysis reveals ectopic RPE covering the dorsal part of the ectopic retina (arrow). Merged channels (C″) showing colocalisation of elongated EGFP+ nuclei and pigmented epithelium (green arrows). (D′) Immunostaining using an anti-GS antibody reveals Muller glia in the endogenous retina and not in the ectopic retina (n=4 medrafish). (D″) Immunostaining using an anti-Rx2 antibody label ectopic photoreceptors (arrow) organised in a mononuclear layer (n=3 medrafish). Scale bars: 100 µm. D, donor; H, host. |
|
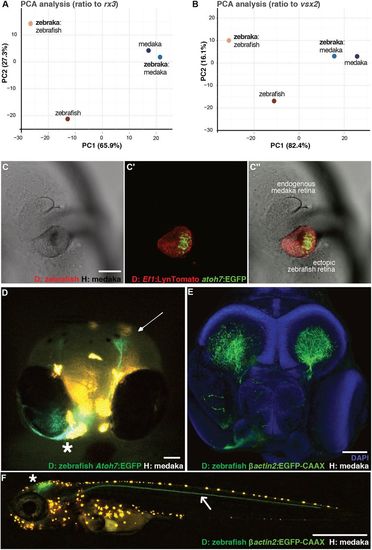
Retinogenesis follows a genetic developmental time. (A,B) PCA analysis of zebrafish and medaka orthologues expressed in the eye during the 24-48 hpf zebrafish developmental time window. The values are FPKM ratios between retinal genes and retinal progenitor markers rx3 (A) and vsx2 (B). (C-C″) Confocal images of the anterior region in a zebraka where the donor is Tg(Ef1:LynTomato, atoh7:EGFP) (C, transmitted; C′, green and red channels; C″, merged image). EGFP expression in the transplanted cluster (C′,C″) is evident at the vesicle stage of the medaka host (n=3 chimeras). (D) Frontal view of a living 5 dpf zebraka where the donor is Tg(Ef1:LynTomato, atoh7:EGFP). Atoh7+ cells from the ectopic zebrafish retina (asterisk) migrate towards the endogenous medaka tectum (arrow) (n=3 transplantation events, n=6 chimeras). (E) Confocal image of a fixed 6 dpf zebraka where the donor is Tg(Olßactin2:EGFP-CAAX) ubiquitously labelling all donor cell projections (n=2 transplantation events, n=6 chimeras). The ectopic retina projects to both host tecti. (F) Confocal image of a living zebraka at 9 dpf. A nerve coming from the ectopic zebrafish retina (located in the contralateral side) navigates along the posterior lateral line nerve (arrow). Other projections from the ectopic retina, presumably from later RGCs, project to the tectum (asterisk) (n=4 transplantation events, n=7 chimeras). Scale bars: 100 µm in C-E; 1 mm in F. D, donor; H, host. Orange dots in D and F correspond to medaka pigments. |
|
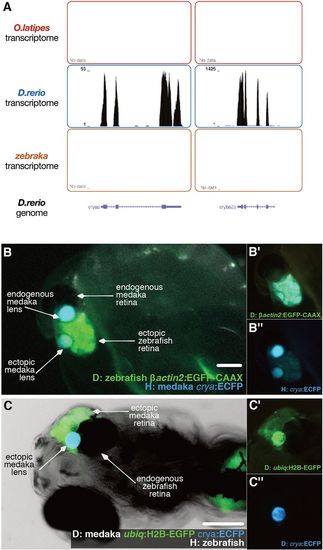
Ectopic zebrafish retina in zebrakas recruits the lens from the medaka host. (A) Transcriptomes of medaka (upper, n=2), zebrafish (middle, n=2) and zebraka (bottom, n=3) plotted along the zebrafish genome. Lens zebrafish genes (crystalin a and crystalin b a2a: cryaa and cryba2a, respectively) are expressed in zebrafish but not in zebrakas. (B-B″) Lateral views of a zebraka where donor Tg(OlBactin2:CAAX-EGFP) cells were transplanted into a Tg(crya:ECFP) host. Merged (B) and single-channel (EGFP in B′, ECFP in B″) images. Cyan expression in the lens of the ectopic retina reveals its host origin (n=3 transplantation events, n=7 chimeras). (C-C″) Ventral views of a medrafish at 5 dpf, where donor Tg(ubiq:H2B-EGFP)(crya:ECFP) cells were transplanted into a non-labelled host. Merged (C) and single-channel (EGFP in C′, ECFP in C″) images. Cyan expression in the lens of the ectopic retina reveals its donor origin (n=10 transplantation events, n=23 chimeras). Scale bars: 100 µm. D, donor; H, host. |