- Title
-
Development of the anterior lateral line system through local tissue-tissue interactions in the zebrafish head
- Authors
- Iwasaki, M., Yokoi, H., Suzuki, T., Kawakami, K., Wada, H.
- Source
- Full text @ Dev. Dyn.
|
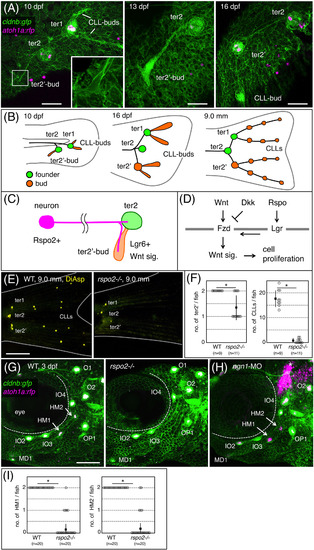
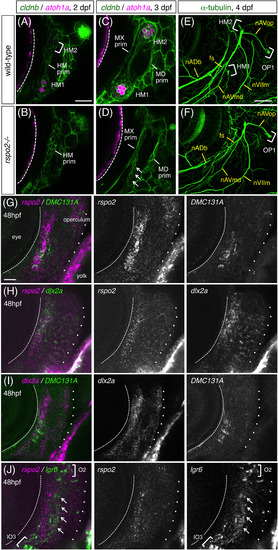
Rspo2 is required for neuromast formation in zebrafish. A, Time‐course observations of the prospective caudal fin region of cldnb:gfp;atoh1a:rfp double transgenic fish at 10, 13, and 16 dpf. Inset shows a higher magnification of the boxed area. B, Schematic drawing for the terminal budding on the caudal fin. C, Proposed model. Rspo2, expressed by sensory neurons, activates Wnt signaling in bud neuromast to promote its proliferation. D, Schematic representation for the regulation of Wnt signaling through its activator (R‐spondin, Rspo) and inhibitor (Dickkopf, Dkk). Fzd, Frizzled; Lgr, Leucine‐rich repeat‐containing G protein‐coupled receptor. E, Caudal fins of wild‐type and rspo2−/− fish stained with DiAsp. F, Quantification of numbers of bud neuromasts in rspo2−/− fish as compared with wild‐type siblings. G, Cranial regions of 3 dpf‐wild‐type and rspo2−/− embryos with cldnb:gfp;atoh1a:rfp background. HM1 and HM2 (arrows in wild‐type) are absent in rspo2−/− embryos. H, 3 dpf‐embryo injected with antisense morpholino against ngn1 (ngn1‐MO). Asterisks indicate the atoh1a:rfp‐expressing hindbrain neurons. I, Quantification of numbers of HM1 and HM2 in rspo2−/− embryos at 4 dpf. Lateral views, anterior is to the left. Data are given as the mean ± SD (F, I) *P < .01 (t‐test), Scale bars: 50 μm (A); 500 μm (E); 100 μm (G) |
|
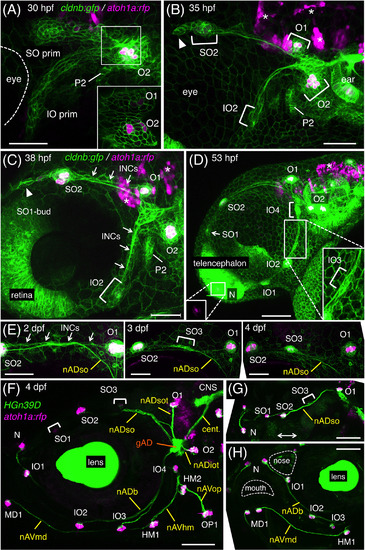
Development of the anterodorsal (AD) system. A, B, Pre‐otic region of cldnb:gfp;atoh1a:rfp embryo at 30 hpf (A) and 35 hpf (B). Two lateral line primordia, SO prim and IO prim, and developing neuromasts are indicated. Arrowhead indicates a cellular extension of the migrating SO prim. P2, the second pharyngeal pouch. Inset, single confocal plane corresponding to the boxed area in A. C, D, Formation of bud and intercalary neuromasts at 38 hpf (C) and 53 hpf (D). SO2 buds off SO1, while IO2 buds off IO1 and N. IO3 and IO4 form through the proliferation of INCs (indicated by arrows in C). Inset in the left bottom indicates atoh1a:rfp‐expression in N. Inset in the right bottom is a higher magnification of IO3. E, Time‐course observation of SO3 formation through the proliferation of INCs. F‐H, Axonal projections to the ALL neuromasts in HGn39D;atoh1a:rfp embryo at 4 dpf. Lateral views, anterior is to the left except for G (dorsal view, the double‐headed arrow indicates the midline of the fish) and H (semi‐frontal view). Scale bars: 50 μm (A‐C, E‐H); 100 μm (D) |
|
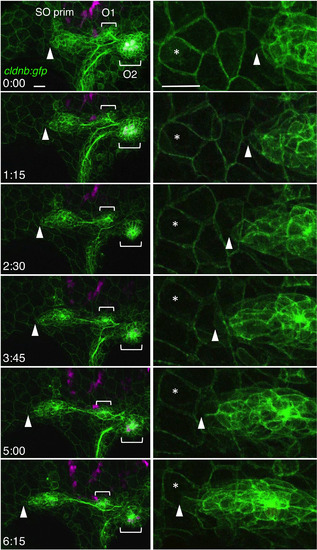
Migration of the SO pim. Images were selected from the time‐lapse recording of cldnb:gfp;atoh1a:rfp embryo around 30‐36 hpf (see Video S1). Right panels show higher magnifications of the leading edge of the primordium (see Video S2). Tip of the primordium with the landmark peridermal cell are indicated by arrowheads and asterisks, respectively. Lateral views, anterior is to the left. Scale bars: 20 μm |
|
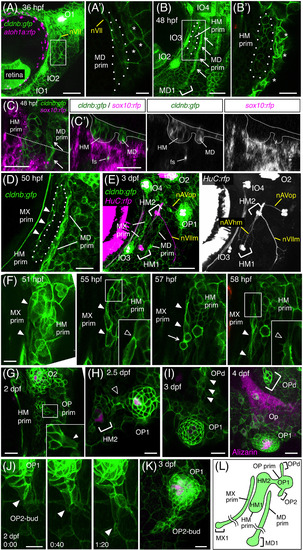
Development of the anteroventral (AV) system. A, Hyoid region of cldnb:gfp;atoh1a:rfp embryo at 36 hpf. A′, Single confocal plane of the boxed area, showing a cluster of elongated cells of the MD prim (dotted line). nVII, facial nerve. Asterisks indicate cldnb:gfp‐expressing peridermal skin cells. B, Elongation of the HM prim at 48 hpf. MD1 forms in the ventral end of the MD prim. B′, Single confocal plane of the boxed area in B. C, Hyoid region of cldnb:gfp;sox10:rfp embryo at 48 hpf. C′, Optical cross‐section along the dashed line in C. fs, facial sensory nerve. D, Formation of the MX prim at 50 hpf. E, Differentiation of HM1 and HM2 in cldnb:gfp;HuC:gal4;UAS:rfp embryo at 3 dpf. F, Budding of the MX prim (indicated by arrowheads) from the HM prim. A single cell elongates (51 hpf), undergoes cell divisions (57 hpf, arrow), and extends ventrally (58 hpf). Insets indicate higher magnifications of the boxed areas, showing detachment of the MX cell from the HM prim. G, Budding of the OP prim from the HM prim at 36 hpf. Inset indicates a higher magnification of the boxed area, showing the budding cell (arrow). H, Differentiation of OP1 at 2.5 dpf. Cells connecting the HM prim and the OP prim are indicated by an open arrowhead. I, Time‐course observation of OPd formation at 3 and 4 dpf. The budding cell is indicated by arrows. Opercle bone (Op) is stained with Alizarin red. J, Time‐lapse observation of OP2 budding at 2 dpf. Arrowheads indicate the leading cell. K, Proliferation of OP2‐bud at 3 dpf. L, Schematic drawing for the developing AV system at 3 dpf. Lateral views, anterior is to the left. Scale bars: 50 μm (A, B, E, K); 20 μm (A′, B′, C, C′, D, G‐I); 10 μm (F, J) |
|
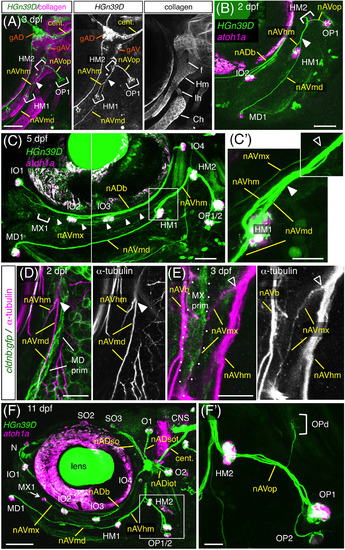
Axonal projections to the anteroventral (AV) neuromasts. A, Axonal projections in 3 dpf‐HGn39D embryo labeled with anti‐type II collagen antibody. Arrowhead indicates the position where nAVmd leaves the nAVhm. Hm, hyomandibular, Ih, interhyal; Ch, ceratohyal cartilages; f, foramen of Hm. B, C HGn39D;atoh1a:rfp embryos at 2 dpf (B) and 5 dpf (C). C′, Higher magnification of the box area in C. Positions where nAVmx and nAVmd leave nAVhm are indicated by open and filled arrowheads, respectively. D, E, cldnb:gfp embryos labeled with the anti‐acetylated α‐tubulin antibody at 2 dpf (D) and 3 dpf (E). The MD and MX primordia are accompanied by nAVmd (D) and nAVmx (E), respectively. F, Axonal projections to the ALL neuromasts in 11 dpf‐HGn39D;atoh1a:rfp. MX1 forms at the anterior end of the nAVmx (arrow). F′, Opercular branches, corresponding to the boxed area in F. Lateral views, anterior is to the left. Scale bars: 50 μm (A‐C, F); 20 μm (C′, D, F′); 10 μm (E) |
|
Expression of rspo2 in hyoid mesenchyme. A‐D, Time‐course observations of wild‐type (A, C) and rspo2−/− (B, D) embryos with cldnb:gfp;atoh1a:rfp background at 2 dpf (A, B) and 3 dpf (C, D). Putative remnants of the HM prim are indicated by arrows in D. E, F, 4 dpf‐wild‐type (E) and rspo2−/− (F) embryos labeled with anti‐acetylated α‐tubulin antibody. fs, facial sensory nerve; nVIIm, facial motor nerve. G, 48 hpf‐DMC131A(rspo2:gal4);UAS:gfp embryo doubly labeled with anti‐GFP antibody and RNA probe for rspo2. H, 48 hpf‐embryo doubly labeled with RNA probes for rspo2 and dlx2a. I, 48 hpf‐DMC131A(rspo2:gal4);UAS:gfp embryo doubly labeled with anti‐GFP antibody and RNA probe for dlx2a. J, 48 hpf‐embryo doubly labeled with RNA probes for rspo2 and lgr6. The posterior margin of the eye is indicated by the dotted line. The operculum is outlined by dots. Lgr6‐expressing cells are indicated by arrows (J). Lateral views, anterior is to the left. Scale bars: 50 μm (E, F); 20 μm (A‐D, G‐J) |
|
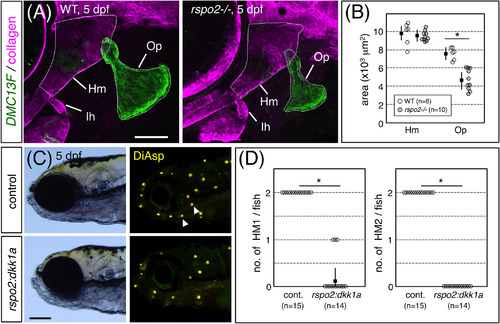
Hyoid‐derived Rspo2 is required for the development of HM1 and HM2. A, 5 dpf‐DMC13F;UAS:gfp embryos, which express GFP in osteoblasts, stained with anti‐type II collagen antibody. Op, opercle. B, Quantification of sizes of Hm and Op defined by the outlines indicated in A. C, 5 dpf‐DMC131A(rspo2:gal4);UAS:dkk1a‐rfp and wild‐type sibling embryos stained with DiAsp. Note the specific loss of HM1 and HM2 (arrowheads). D, Quantification of numbers of HM1 and HM2 in wild‐type and dkk1a‐expressing embryos. Lateral views, anterior is to the left. Data are given as the mean ± SD (B, D) *P < .01 (t‐test). Scale bars: 100 μm (C); 50 μm (A) |
|
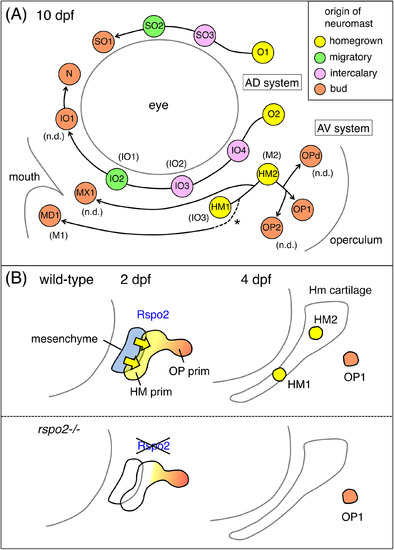
Model for the development of the ALL neuromasts in zebrafish. A, Schematic representation for the ALL neuromasts at 10 dpf. The origin of each neuromast is indicated by a different color. The formation of MD1 by budding is presumptive (dotted line with an asterisk). Designations by Raible and Kruse14 are indicated in parentheses. “n.d.” indicates the neuromasts not described in the previous study. B, Model for the role of Rspo2 during the neuromast formation. Rpso2 (yellow arrows), emanated by the hyoid mesenchyme (blue), acts on the adjacent HM prim to promote proliferation and differentiation of HM1 and HM2 (yellow). Budding of OP1 (orange) from the HM prim takes place normally |