- Title
-
A novel role for zebrafish zic2a during forebrain development
- Authors
- Sanek, N.A., and Grinblat, Y.
- Source
- Full text @ Dev. Biol.
|
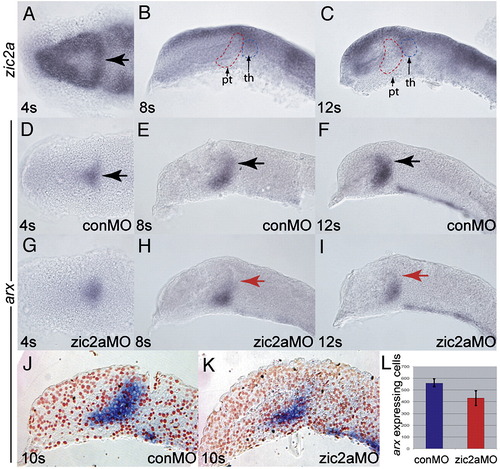
Zic2a is required for maintenance, but not for initiation of arx expression in the PT primordium. Embryos were stained by ISH for expression of zic2a (A–C) or arx (D–I). (A) Uninjected embryos express zic2a transiently in the early PT at 4 somites. (B, C) zic2a is not expressed in the PT at 8 and 12 somites. Prethalamus (pt) and thalamus (th) are outlined for reference. (D, E) Normal arx expression in the PT primordium of control MO injected embryos at 4, 8 and 12 somites. (G, H) arx expression in zic2a morphants. (G) arx is expressed normally at 4s (44/50 embryos, 3 exp.). (H) arx is mildly reduced at 8 somites (8/23 embryos, 2 exp.). (I) arx is drastically reduced at 12 somites (28/33 embryos, 2 exp.). (J, K) Representative parasagittal sections of conMO- and zic2aMO-injected embryos stained for arx by ISH at 10s. (L) Graph of average number of arx expressing cells in conMO (n = 3) and zic2aMO-injected (n = 3) embryos (results significant at p = 0.05). All embryos are shown with anterior to the left. Panels A, D and G are dorsal views, all others are lateral views. Arrows mark the PT primordium. EXPRESSION / LABELING:
PHENOTYPE:
|
|
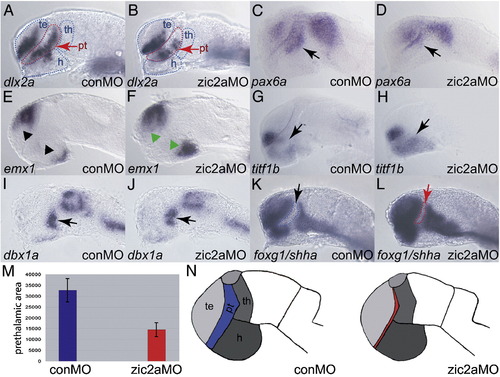
Zic2a functions primarily in the PT during forebrain development. The effect of Zic2a depletion on overall pattern in the forebrain at Prim-5 was examined using ISH with several markers of forebrain subdivisions (see Supplementary Table 1 for numbers). (B) dlx2a expression is reduced in the PT, but not affected in the telencephalon of zic2a morphants. (C, D) pax6a expression is reduced in the PT region of Zic2a depleted embryos (arrows), but not affected in the telencephalon. (E, F) Expression of emx1, a marker of posterior telencephalon and posterior hypothalamus is expanded in both domains. (G, H) titf1b expression in the hypothalamus is expanded anteriorly in zic2a morphants. (I, J) The thalamus, marked by dbx1a, is formed normally. (K, L) Expression of foxg1 in the telencephalon and shha in the ZLI are normal in zic2a morphants. Note that the AD area, bordered by expression of foxg1 anteriorly and shha posteriorly, is reduced in zic2a morphants. (M) The AD area was measured in pixels2 using Axiovision software (Zeiss). The bar graphs represent average AD areas calculated from 12 conMO injected embryos and 18 zic2aMO injected embryos. Standard error bars shown, results significant at p = 0.001. (N) Summary of the effects of Zic2a depletion on forebrain regionalization. The strongest defect is observed in the AD, indicated in red. All embryos are at Prim-5 stage and are shown in lateral views with anterior to the left. Arrows mark the PT and arrowheads mark expanded domains in the telencephalon and hypothalamus. te = telencephalon, th = thalamus, pt = prethalamus, h = hypothalamus. |
|
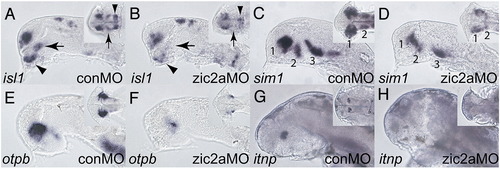
Zic2a is required for diencephalic neurogenesis. The effect of Zic2a depletion on neurogenesis in the forebrain was examined using ISH with neuronal markers. (A, B) isl1 expression in zic2a morphants at Prim-5 shows loss of the ventrorostral cluster (VRC, arrow), and a fusion of the dorsorostral cluster (DRC, arrowhead) (35/39 embryos, 2 exp.). (C, D) sim1 expression in the preoptic area is strongly reduced (27/36 embryos, 2 exp.) at Prim-5. (E, F) otpb expression in the preoptic area is also strongly reduced (30/30 embryos, 2 exp.). (G, H) Expression of itnp, a marker of differentiated neurons at 2 dpf, is lost in zic2aMO injected embryos (28/34 embryos, 2 exp.). Embryos are shown in lateral view with anterior to the left. Insets are ventral views of the same embryos, except in panels A and B, which are anterio-ventral views. Numbers in panels C and D mark different neural clusters. |
|
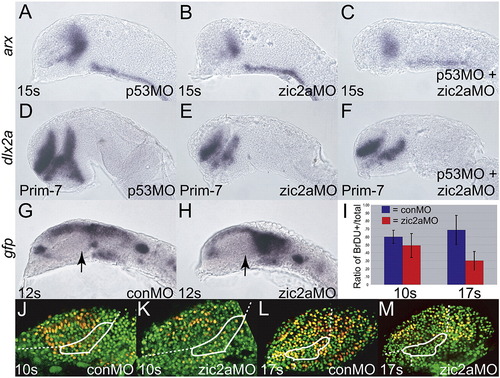
Zic2a regulates proliferation, but not apoptosis or differentiation of PT precursors. Embryos were injected singly or co-injected with zic2aMO and p53MO and stained out for expression of arx, an early PT marker at 15 somites (A–C), dlx2a, a late PT marker, at Prim-7 (D–F), or gfp (G, H). (A) p53 morphants show normal expression of arx (26/26 embryos, 2 exp.). (B) Embryos injected with zic2aMO alone show a strong reduction of arx expression at 15s (13/17 embryos, 2 exp.). (C) Co-injection of zic2aMO and p53MO leads to a similar reduction of arx expression (11/16 embryos, 2 exp.). (D) p53MO-injected embryos show no patterning defect at Prim-7 (29/30 embryos, 2 exp.). (E) zic2aMO-injected, or (F) zic2aMO and p53MO co-injected embryos show equivalent loss of dlx2a expression at Prim-7 (19/26 embryos, 2 exp. and 33/45 embryos, 2 exp. respectively). (G, H) Transgenic Tg(HuC:gfp) embryos express gfp in post-mitotic neurons. (G) conMO-injected transgenic embryos show no evidence of gfp-positive post-mitotic cells in the PT at 12s. (H) zic2aMO morphants do not contain prematurely differentiating cells in the PT at 12s (17/17 embryos, 3 exp.). (I) Ratios of BrdU positive cells/total cells in conMOs and zic2aMOs at 10s and 17s. 10s analysis revealed no significant difference between conMO-injected (n = 5) and zic2aMO-injected (n = 4) embryos. 17s analysis showed a significant difference (p = 0.005) between conMOs (n = 5) and zic2aMOs (n = 4). (J–M) Representative confocal sections of MO-injected embryos, showing BrdU-positive cells in yellow and BrdU-negative nuclei in green. White outlines the approximate prethalamic area determined by arx expression at the same stages. Embryos are shown in lateral view, anterior to the left. Arrows mark the PT. EXPRESSION / LABELING:
PHENOTYPE:
|
|
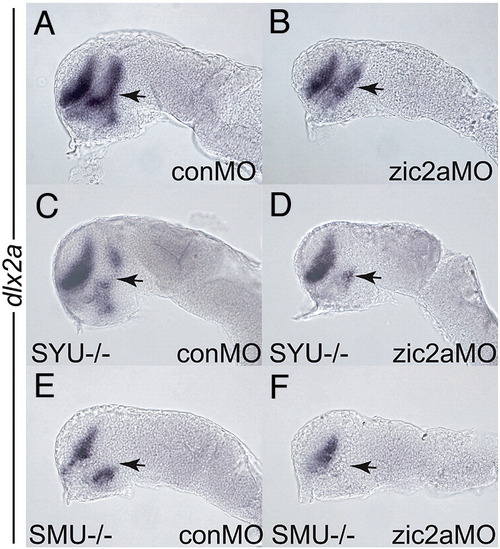
Zic2a promotes PT formation in cooperation with components of the hedgehog signaling pathway. Embryos of different genetic backgrounds were injected with a conMO (A, C, E) or a zic2aMO (B, D, F) and stained for dlx2a expression by ISH at the prim-5 stage. (A, B) Wild type embryos depleted of Zic2a (B) show a typical reduction of PT relative to control morphants (A). (C) Homozygous syut4 mutant embryos show reduction of PT dlx2a (9/35 embryos, 2 exp.). (D) syut4 mutants depleted of Zic2a exhibit an almost complete loss of dlx2a in the PT (17/74 embryos, 3 exp.). (E) homozygous smob641 embryos show reduced dlx2a expression in the PT (25/83 embryos, 3 exp.). (F) smob641 mutants depleted of Zic2a show complete loss of dlx2a in the PT (60/195 embryos, 4 exp.). Embryos are shown in lateral view, anterior to the left. Arrows point to the PT. |
|
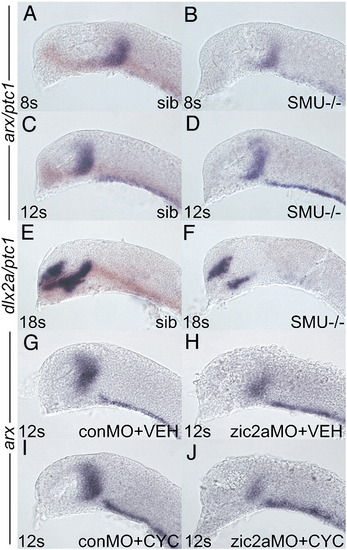
Zic2a acts before hedgehog signaling to promote maturation of the PT primordium. Embryos of different genetic backgrounds were injected with conMO or zic2aMO and stained for arx expression by ISH, except in panels E, F, which were stained for dlx2a. (A–F) Embryos were derived from a smob641/+ incross. Wildtype and heterozygous siblings were identified by the presence of ptc1 expression (orange), while mutant embryos lacked any ptc1 expression. (A) Wildtype sibling embryos (84/117) have very similar arx expression as (B) mutant siblings (33/107 embryos) at 8s. (C) Wildtype embryos at 12s (70/95 embryos) are indistinguishable from (D) mutant siblings (25/95 embryos). (E) At 18s, dlx2a is strongly expressed in the PT of wildtype embryos (109/143 embryos). (F) Mutant embryos display a dramatic reduction of dlx2a expression by 18s (23/132 embryos). (G, H) Embryos were injected with either conMO (G, 13/13 embryos) or zic2aMO (H, 7/7 embryos), treated with vehicle at 50–60% epiboly and fixed at 12s. (I, J) Embryos were injected with conMO (I, 12/12 embryos) or zic2aMO (J, 8/8 embryos), treated with 10 μM cyclopamine at 50–60% epiboly and fixed at 12s. Embryos are shown in lateral view, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
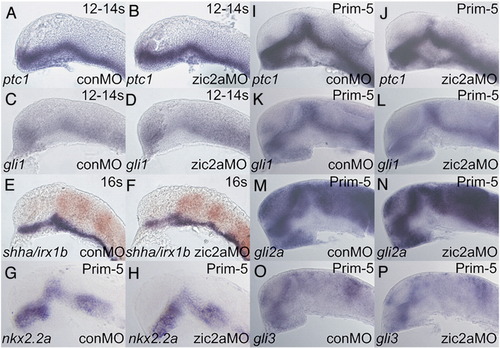
Zic2a controls PT patterning independent of hedgehog signaling. Embryos were injected with conMO or zic2aMO, and examined by ISH for expression of the following markers, which were expressed correctly in zic2a moprhants. (A, B) ptc1 at 12–14s (20/20 morphants, 2 exp.). (C, D) gli1 at 12–14s (9/9 morphants, 2 exp.). (E, F) shha at 16s (38/38 morphants, 2 exp.). (G–P) Embryos are at Prim-5 (see Supplementary Table 1 for numbers). (G, H) nkx2.2a. (I, J) ptc1. (K, L) gli1. (M, N) gli2a. (O, P) gli3. Embryos are shown in lateral views, anterior to the left. EXPRESSION / LABELING:
|
|
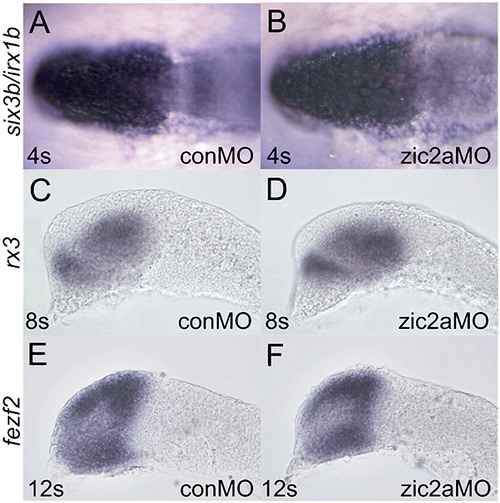
Forebrain patterning in zic2a morphants at 4s–12s. (A, B) six3b, which marks the eye field and irx1b, which marks the future thalamus, are not affected at 4s (46/47 morphants, 3 exp.). (C, D) rx3, another marker of the eye field and the anterior hypothalamus is not affected at 8s (31/32 morphants, 2 exp.). (E, F) fezf2 marks the forming prethalamus and is not affected at any time between 4s and 12s (12s stage shown, 24/25 morphants, 2 exp.). Embryos are shown in lateral views, anterior to the left. EXPRESSION / LABELING:
|
|
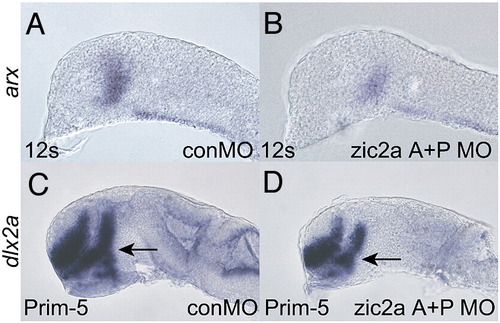
Non-overlapping zic2aMOs cause similar patterning defects. (A, B) Embryos co-injected with translation-blocking MOs designed against the proximal promoter (zic2a P MO) and the first AUG (zic2a A MO) show similar arx reductions at 12s (4/20, 1 exp.) as embryos injected with splice-blocking zic2aMO (see Fig. 1). (C, D) Prim-5 stage embryos injected with zic2a A + P MO also show reduced dlx2a expression (14/45, 2 exp.) similar to zic2aMO-injected embryos (see Fig. 2). EXPRESSION / LABELING:
PHENOTYPE:
|
|
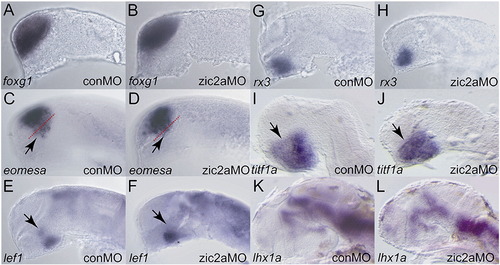
Forebrain patterning in zic2a morphants. (A, B) foxg1 expression in the telencephalon is not affected in zic2a morphants. (C, D) eomesa expression is normal in the telencephalon, but is reduced in anterior diencephalon (dashed red lines demarcate border between telencephalon and diencephalon). (E, F) lef1 expression is reduced in the PT region of Zic2a depleted embryos. (G, H) rx3 expression is not affected in the anterior hypothalamus, however (I, J) titf1a is weakly expanded anteriorly. (K, L) lhx1a expression is not affected in zic2a morphants at 2 dpf. Embryos are shown in lateral views, anterior to the left. All numbers for these experiments can be found in Supplementary Table 1. |
|
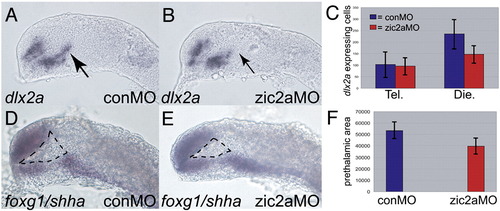
PT defects in zic2a morphants at 17–18s. (A, B) dlx2a expression in the PT is reduced in Zic2a-depleted embryos (49/80 embryos, 3 exp.). (C) Graph showing average numbers of dlx2a-expressing cells in the telencephalon and diencephalon of conMO injected (n = 5) and zic2aMO injected (n = 9) embryos stained for dlx2a at 17–18s. Embryos were sectioned (see methods) to obtain accurate counts of dlx2a-expressing cells. There was no difference in number of telencephalic dlx2a-expressing cells between control and Zic2a morphants. However, there were significantly fewer diencephalic dlx2a-expressing cells in zic2aMOs compared to conMOs (p = 0.01). (D, E) foxg1 and shha expression was used to deliniate the prethalamic area at 17–18s. The dotted lines in D and E outline the prethalamic area. (F) Graph representing the average prethalamic area (pixels2) in conMOs (n = 10) and zic2aMOs (n = 8). Results are significant at p = 0.001. Embryos are shown in lateral views, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
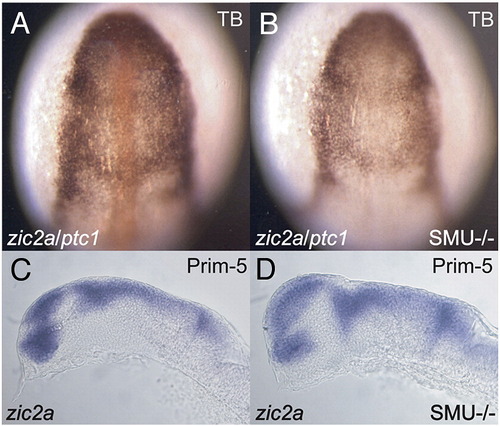
Zic2a expression is unaffected by loss of hedgehog signaling. (A, B) Double ISH for zic2a and ptc1 expression in smob641/+ incross progeny at tail-bud stage (TB). (B) Homozygous null embryos were identified by lack of ptc1 expression. (C, D) ISH for zic2a expression in smob641/+ incross progeny at Prim-5 stage. Homozygous null embryos were identified morphologically. |

Unillustrated author statements EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Developmental Biology, 317(1), Sanek, N.A., and Grinblat, Y., A novel role for zebrafish zic2a during forebrain development, 325-335, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.