- Title
-
Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3
- Authors
- Stigloher, C., Ninkovic, J., Laplante, M., Geling, A., Tannhauser, B., Topp, S., Kikuta, H., Becker, T.S., Houart, C., and Bally-Cuif, L.
- Source
- Full text @ Development
|
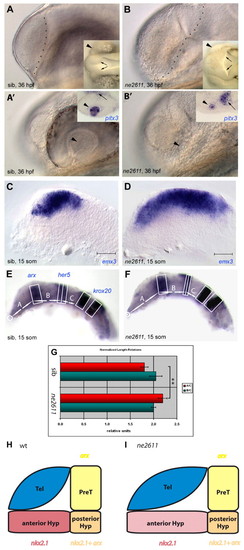
Enlarged telencephalon and lack of retina in ne2611. All views are lateral, anterior left. (A-B') Compared morphology of ne2611 (B,B') and wild-type siblings (sib; A,A') at 36 hpf; views with parasagittal and lateral focus, respectively. (A,B) Note the enlarged telencephalon, delimited by a dashed line, in ne2611. Insets are dorsal views of the same embryos showing absence of the retina (arrowheads) and an expanded diencephalic ventricle (arrows) in ne2611. (A',B') Note the maintenance of a lens (arrowhead) in ne2611, albeit smaller than in wild-type siblings. Insets show expression of pitx3, molecularly identifying the lens (arrowheads) in both genotypes. The adjacent domain of pitx3 expression (small arrows) is hypothalamic, it is displaced towards the lens in ne2611 due to the absence of eyes. (C,D) Compared expression of the telencephalic marker emx3 at the 15-somite stage in ne2611 (D) and wild-type siblings (C); scale bar: 0.02 mm. The mutants display a massive anteroposterior enlargement of the emx3 domain. (E-G) Relative anteroposterior sizes of the anteriormost forebrain (domain A) versus prethalamus, thalamus, pretectum and midbrain (domain B) at 15 somites in ne2611 (E) compared with wild-type siblings (F). (G) Measurements are normalized to the size of the anterior hindbrain (domain C), and the domains are defined with the genes indicated in E. Bars indicate s.e.m. The difference in A/C length between wild type and ne2611 is statistically significant (two-sample independent Student's t-test, P values are given in the text). (H,I) Schematic representation of the size of the different anterior forebrain territories in wild type versus ne2611 siblings at 15 somites. The genes used as landmarks are color coded (yellow: arx only, prethalamus; red: nkx2.1 only, anterior hypothalamus; orange: arx+nkx2.1, posterior hypothalamus). Prethalamus and posterior hypothalamus are unchanged in the mutants. The anterior hypothalamus appears elongated, but might be simply stretched (hence the lighter red color) by the anteroposterior enlargement of the telencephalon. Later, the anterior hypothalamus appears reduced or missing (see also Fig. S1I,J in the supplementary material). EXPRESSION / LABELING:
|
|
The eye field is partially specified but is not maintained in ne2611 mutants. All views, anterior left. (A,B) Expression of otx2, labeling the wild-type retina (A, blue arrow) but not the telencephalon, is absent in the enlarged telencephalon of ne2611 sibling embryos (B). Lateral views; t, optic tectum; telencephalon (arrowhead) delimited by gray dots in B. (C-H) Expression of the earliest eye-field marker rx3 (C-E; dorsal views of flat-mounted embryos), and of rx1 (F-H; lateral views of whole embryos), is initiated normally in ne2611. (I-K) Expression of rx2, normally detected at 3-somites in wild type (K, inset), is never initiated in ne2611 (K). Lateral views of whole embryos. |
|
ne2611 is a new null allele of rx3. (A) Sequencing trace data of rx3 cDNA from a ne2611 mutant (right) and a wild-type sibling (left) reveals a T to C transition (arrows). (B,C) Rx3 protein sequences (B) and structures (C) in wild type (+) and ne2611 mutants. The ne2611 mutation leads to a Serine to Proline exchange within the Rx3 homeodomain (asterisk in C). Dark gray box, octapeptide; red box, homeodomain; light gray box, otparistaless-rx domain. (D-F) Expression of the telencephalic marker emx3 in wild-type (D), ne2611 (E) and ckhs399 (F) embryos at 48 hpf (lateral views, anterior left; scale bar: 0.02 mm). ckhs399 embryos display a telencephalic expansion similar to that of ne2611 mutants. (G-K) Embryos from a ne2611/+ ne2611/+ cross were injected at the one-cell stage with BAC CHORB736A01233Q containing the rx3 locus and are observed at 24 hpf. (I,J) Representative injected ne2611 mutants; note the restoration of the retina compared with uninjected wild type (G) or ne2611 (H; small lens in H indicated by the white arrow). (K) Percentage of embryos lacking eyes after BAC injection compared with non-injected embryos. BAC injection restored retinal development in 79% of mutant embryos. (L,M) Phenotypes triggered by ectopic expression of wild-type rx3 versus rx3ne2611 mRNA. Wild-type embryos were injected at the one-cell stage and observed at 24 hpf (lateral views, anterior left). Ectopic expression of rx3 causes head truncations (L, arrow; compare with wild type, inset) whereas rx3ne2611 has no effect (M). (N) Percentage of embryos showing reduction of tlc expression (left two bars) (see Fig. S2 in the supplementary material) or head truncation (right two bars) following injection of rx3 or rx3ne2611 mRNA (as indicated). EXPRESSION / LABELING:
|
|
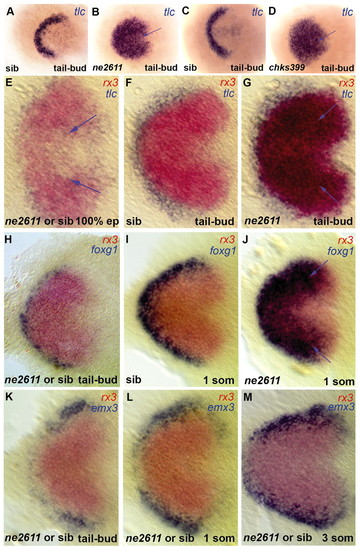
Rx3 controls early patterning of the presumptive anterior forebrain. Expression of the markers indicated (color coded) in ckhne2611 (B,G,J), ckhs399 (D) or their wild-type siblings (A,C,F,I); E,H,K-M are non-genotyped embryos from crosses between ckhne2611/+ heterozygotes. All views are dorsal anterior left in whole embryos (A-D) or flat mounts (E-M). (A-D) tlc expression identifies the presumptive telencephalon at tail-bud stage and is massively expanded in both ckhne2611 (B) and ckhs399 (D) mutants compared with their wild-type siblings (A,C). (E-G) No difference in tlc expression is observed between wild-type and mutant embryos at 100% epiboly, when tlc expression is downregulated from the presumptive eye and hypothalamus fields, labeled by rx3 (see the few remaining tlc-positive cells in the rx3-positive domain at 100% epiboly; arrows in E, absent in F). At tail-bud stage the ectopic tlc expression overlaps the rx3 domain (G, blue arrows). (H-J) Expansion of foxg1 across the eye field is absent at tail-bud stage (H) but is visible at the one-somite stage (J, blue arrows). (K-M) The relative expression of emx3 and rx3 are not noticeably affected in mutants before the 3-somite stage. EXPRESSION / LABELING:
|
|
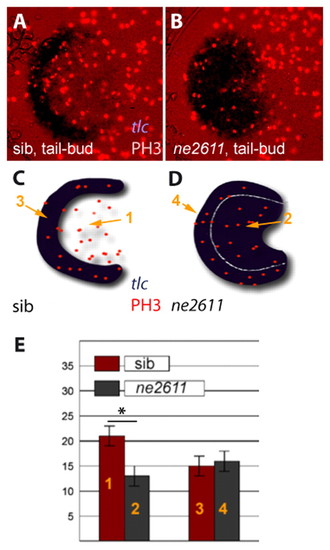
Rx3 accounts for the higher proliferation of eye field versus telencephalic precursors. (A,B) Immunodetection of the M-phase marker Phosphohistone-H3 (red nuclei) in the anterior neural plate of ckhne2611 mutants (right panel) and their wild-type siblings (left panel) at tail-bud stage (dorsal views, anterior left). tlc expression (ISH, blue) serves as a marker of the presumptive telencephalon in wild-type embryos and of the telencephalon+eye field in ckhne2611. (C,D) Schematic representation of the embryos in A,B to localize the presumptive telencephalic and eye fields in wild-type embryos (domains 3 and 1, respectively) and the corresponding territories in ckhne2611 siblings (domains 4 and 2, respectively) at tail-bud stage. (E) Number of PH3-positive cells in domains 1-4 (see C,D) in wild-type (red) versus ckhne2611 (gray) embryos. Bars indicate standard errors. Proliferation is significantly decreased in the presumptive eye field, but is unaltered in the presumptive telencephalon, in ckhne2611 compared with wild-type embryos at tail-bud stage (two-sample independent Student's t-test, P values are given in the text). EXPRESSION / LABELING:
|
|
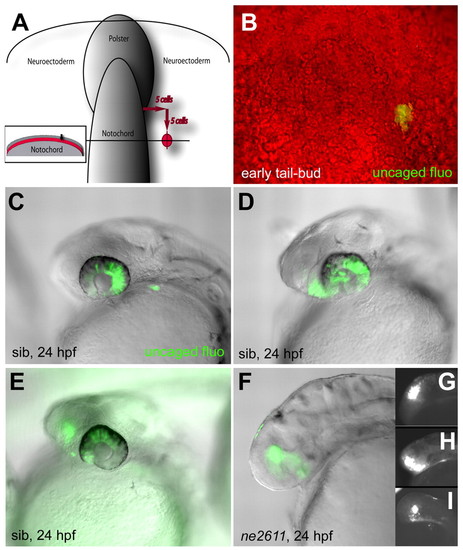
Cells of the presumptive eye field acquire a telencephalic identity in the absence of Rx3. (A,B) Location of uncaged cells compared with the notochord, polster and enveloping layer (inset in A) at the early tail-bud stage, and photomicrograph of the fluorescent clone immediately following uncaging (B) (dorsal views, anterior up). (C-E) Fate of the uncaged progeny in wild-type embryos at 24 hpf (overlay of fluorescence and Nomarski optics views, anterior left). (C) Retina only (45% of cases); (D) retina and anterior hypothalamus (21% of cases); (E) retina and telencephalon (31% of cases) (see also Table 1). (F-I) Fate of the uncaged progeny in ckhne2611 embryos at the 24 hpf stage (overlay of fluorescence and Nomarski optics views in F, fluorescence only in G-I, anterior left). All clones contribute to the telencephalon (100% of cases, see Table 1). |
|
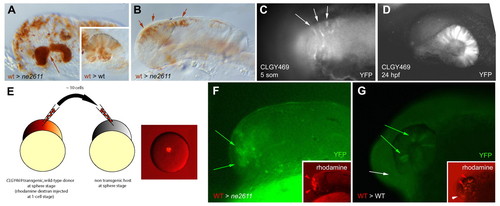
Rx3 controls eye field versus telencephalic fate in a cell-autonomous manner. (A,B) Transplantation of wild-type cells (brown) into the anterior forebrain of a ckhne2611 or wild-type (inset) host. (A) When a large number of cells are transplanted, retinal structures are rescued and evaginate (arrow). They are entirely composed of wild-type cells, whereas transplanted cells distribute randomly in a wild-type host (inset). (B) When a low number of cells is transplanted, retinal evagination does not occur. (C,D) Expression of YFP (revealed by anti-GFP immunocytochemistry) in transgenic CLGY469 embryos before (C; dorsal view) and after (D; lateral view) retinal evagination. YFP expression is restricted to eye-field cells and their descendants. It is absent in ckhne2611 mutants (see text). (E-G) Transplantation of a few wild-type cells transgenic for CLGY469 into the animal pole of a ckhne2611 (F) or wild-type (G) host. In a mutant host (F), retinal evagination does not occur (as in B), but expression of the transgene (green) is rescued in some transplanted cells (red, inset), indicating rescue of the eye-field fate. In a wild-type host, transplanted cells contribute to the retina (red arrowheads) and telencephalon (white arrowhead; G, inset), but only retinal cells express the transgene (G, green arrows, as opposed to white arrow). |
|
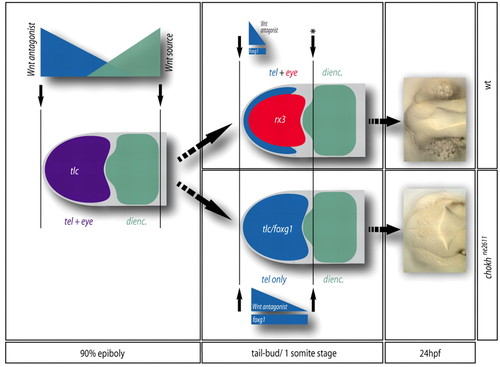
Model for the segregation of the telencephalic and eye anlage during zebrafish forebrain patterning. Patterning boundaries are indicated by black arrows. During gastrulation (left panel), a prevalent view is that the forebrain is patterned as a whole by the opposite activities of a posterior Wnt source located at the level of the posterior diencephalon (green gradient) and Wnt antagonists (purple gradient) located at the ANB (Wilson and Houart, 2004). `High Wnt' defines the presumptive diencephalon (green), whereas `low Wnt' defines an anterior domain (purple) including the presumptive telencephalon and eyes. At the tail-bud stage (middle panel), a posterior patterning boundary (black arrow with star) is set-up in front of the diencephalon, isolating the anterior forebrain. Within the latter domain, Rx3 activity (red) represses tlc expression (blue gradient) and foxg1 (blue bar) and attributes retinal fate to its expressing cells at the expense of a telencephalic fate, thereby segregating retinal and telencephalic identities (top panel). In the absence of Rx3, retinal precursors acquire a telencephalic fate (bottom panel), leading at 24 hpf to the absence of eyes and an enlarged telencephalon (right panel). |
|
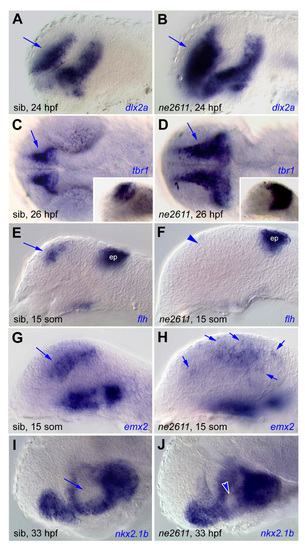
Local patterning defects in the telencephalon and hypothalamus, in ne2611 mutants compared with their wild-type siblings. (A,C,E,G,I) Wild-type siblings; (B,D,F,H,J) ne2611 mutants. All views are lateral (except for C,D: dorsal views), anterior left, at the stages indicated. In ne2611 mutants, the expression domains of the ventral and proximal telencephalic markers dlx2a (A,B) and tbr1 (C,D, insets are lateral views of the same embryos) are enlarged but normally organized, the telencephalic but not epiphyseal (ep) expression of flh is absent (E,F, arrowhead) and that of emx2 is disorganized (G,H, arrows). The anterior hypothalamus, revealed by nkx2.1b (arrows in I) is reduced (arrowheads in J). EXPRESSION / LABELING:
|
|
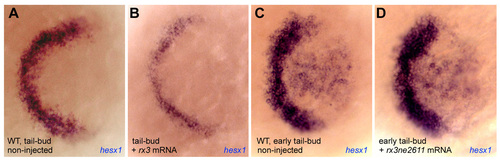
Fig. S2. Expression of the early telencephalic marker hesx1. (A-D) Expression of the early telencephalic marker hesx1 in wild-type embryos (A,C) compared with embryos injected with rx3 (B) or rx3ne2611 (D) mRNA at the stages indicated. All views are dorsal, anterior left. Note that rx3, but not rx3ne2611, reduces hesx1 expression. An identical result is obtained when probing for tlc expression (not shown). EXPRESSION / LABELING:
|