- Title
-
Cadherin-16 regulates acoustic sensory gating in zebrafish through endocrine signaling
- Authors
- Schloss, S.S., Marshall, Z.Q., Santistevan, N.J., Gjorcheska, S., Stenzel, A., Barske, L., Nelson, J.C.
- Source
- Full text @ PLoS Biol.
|
|
|
|
|
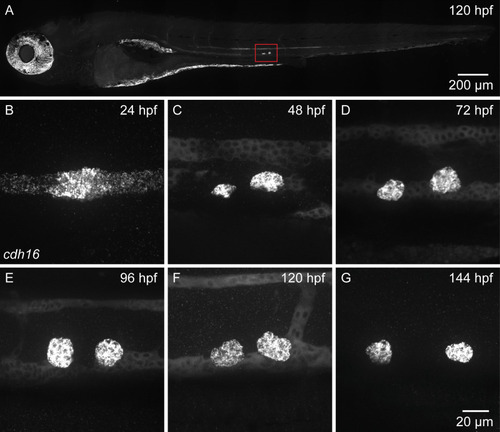
Ubiquitous expression of |
|
|
|
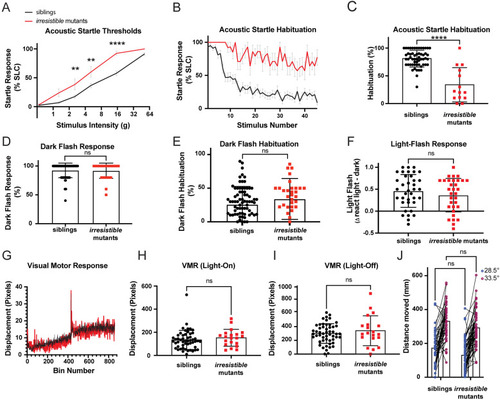
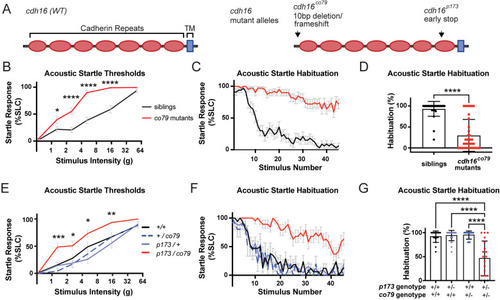
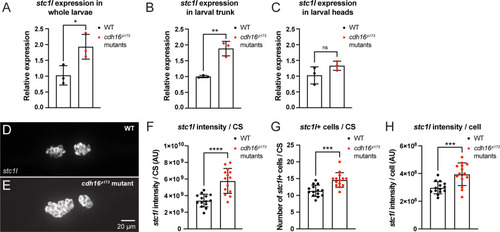
Cadherin-16 suppresses |
|
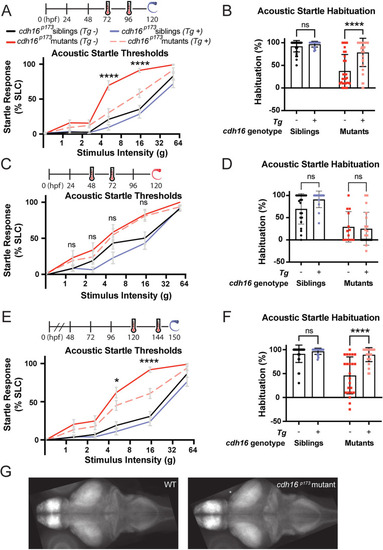
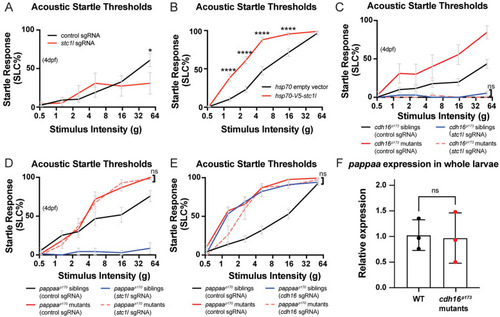
Cadherin-16 promotes startle thresholds by limiting Stanniocalcin 1l expression and promoting Papp-aa function. |
|
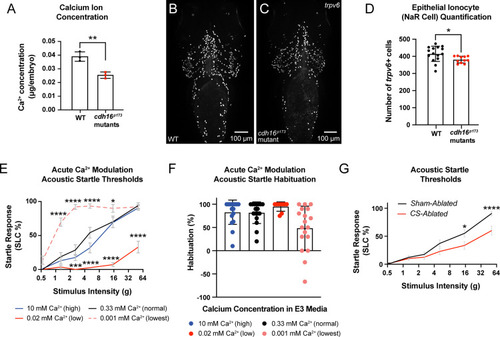
The corpuscles of Stannius (CS) and Ca2 |
|
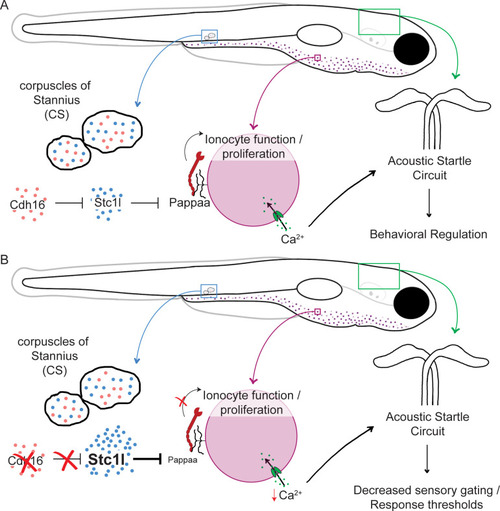
Proposed model. |