- Title
-
The lh3 Glycosyltransferase Directs Target-Selective Peripheral Nerve Regeneration
- Authors
- Isaacman-Beck, J., Schneider, V., Franzini-Armstrong, C., Granato, M.
- Source
- Full text @ Neuron
|
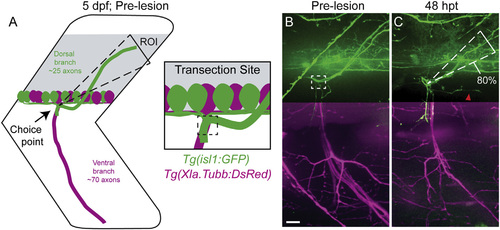
Regenerating Motor Axons Select Their Original Trajectory with High Fidelity (A and B) A schematic (A) and in vivo imaging (B) demonstrate that zebrafish peripheral motor axons traverse a common path then diverge to innervate functionally distinct myotomal regions (dashed triangle, dorsal ROI; dashed boxes, nerve transection site; scale bar, 10 µm). (C) By 48 hpt, dorsal axons regrow with great fidelity to the original trajectory (80% of fascicles that developed in the dorsal ROI regrew on the dorsal path; n = 14 larvae, 26 nerves; red arrowhead, misguided regrowth). In (B) and (C), we omitted Tg(Xla.Tubb:DsRed) signal from the dorsal panel as expression in the spinal cord overwhelms the max projection image. The ventral panel shows expression from both transgenes. |
|
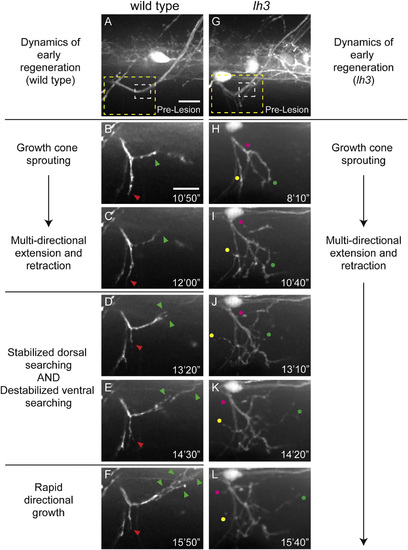
lh3 Is Required for Pathway Stabilization in Early Regeneration (A) Wild-type dorsal nerve prior to nerve transection (white dashed box, transection site; yellow dashed box, region magnified in B–F; scale bar, 10 µm). (B and C) Regenerating wild-type dorsal growth cones (B) and probe the injury gap (C) through multi-directional extension and retraction (red arrowhead, ventral probing; green arrowhead, dorsal probing; scale bar, 10 µm). (D–F) Axons then destabilize non-dorsal searching and stabilize dorsal searching at <13 hpt (D) to ~14 hpt (E) leading to rapid directional growth (F; n = 8 larvae, 15/16 nerves). (G) Conditional lh3 mutant dorsal nerves develop indistinguishably from wild-type (white dashed box, transection site; yellow dashed box, region magnified in H–L). (H–L) In the absence of lh3, axons sprout growth cones (H) after transection. Axons probe the myotome multi-directionally at 10 hpt (I), 13 hpt (J), 14 hpt (K), and 15 hpt (L; magenta, yellow and green dots track individual fascicles) but fail to stabilize dorsal searching and destabilize ventral searching (n = 11 larvae, 10/26 nerves). When lh3 mutant axons stabilized growth, these axons often grew on non-dorsal paths (n = 8/26 nerves, p < 0.001). |
|
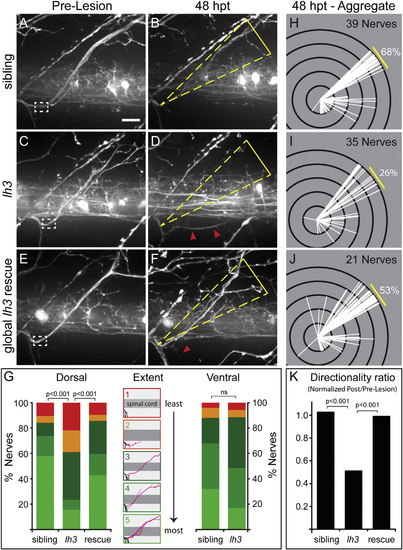
lh3 Is Required for Regenerative Axonal Growth and Target-Selective Regeneration (A–D) Examples of pre-lesion (A) and 48 hpt (B) wild-type motor nerve show robust and directional regrowth. In contrast, pre-lesion (C) and 48 hpt (D) conditional lh3 mutant motor nerve examples show diminished regrowth that often targeted non-dorsal regions (white dashed box, transection site; yellow triangle, dorsal ROI; red arrowheads, misguided fascicles; scale bar, 10 µm). (E and F) lh3 expression after transection rescued this defect; compare pre-lesion (E) to 48 hpt (F) examples. (G) lh3 is required for regenerative growth across populations (wild-type sibling, n = 13 larvae, 39 nerves; lh3, n = 13 larvae, 35 nerves; global lh3 rescue, n = 8 larvae, 21 nerves). Camera lucida tracings of regrowth “extent” categories described in Supplemental Experimental Procedures (black, uninjured axons; pink, regenerated axons). Regenerating ventral axons do not require lh3 function (sibling, n = 9 larvae, 25 nerves; lh3, n = 16 larvae, 35 nerves). (H–K) Modified Sholl analysis reveals that in comparison to siblings (H), fewer lh3 fascicles (I) regrew to the dorsal myotome. This defect was partially rescued by ubiquitous lh3 transgene expression during regeneration (J). (K) These differences were statistically significant after adjusting for developmental dorsal axon patterning in the directionality ratio. |
|
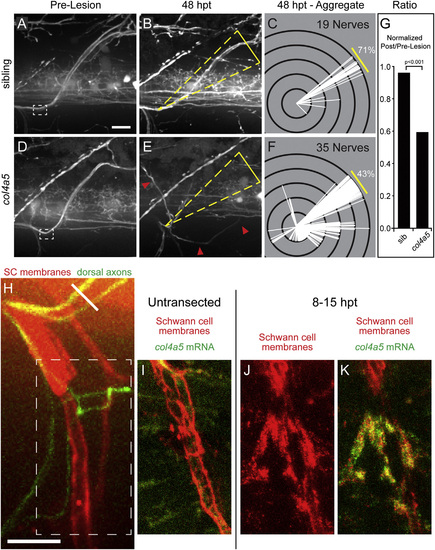
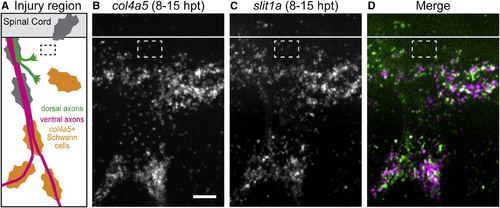
The lh3 Substrate collagen4a5 Is Upregulated after Nerve Transection and Directs Regenerating Dorsal Nerve Axons (A–F) Compared to pre-lesion (A), 48 hpt sibling nerves (B) regenerate to the original outgrowth pathway. Modified Sholl analysis (C; n = 7 larvae, 19 nerves) reveals that the majority of sibling nerves regenerate on the correct path. In contrast, pre-lesion (D) and 48 hpt (E) col4a5 nerve examples and Sholl analysis (F; n = 12 larvae, 35 nerves) show that mutant nerves frequently regrow into aberrant regions of the myotome (yellow triangles, dorsal ROI; red arrowheads, misguided fascicle). (G) These differences were statistically significant after adjusting for developmental dorsal axon patterning in the directionality ratio. (H) Region of transected nerves showing col4a5 mRNA signal in (J) and (K) (oblique white line, transection site; white dashed box, region of nerve shown in I–K). (I) col4a5 in situ hybridization in untransected hemisegments revealed sparse signal (n = 12 larvae, 36/54 nerves). (J and K) 8–15 hr post-transection, col4a5 mRNA (J) was upregulated in Schwann cells (K) ventral and ventrolateral to the transection site (n = same 12 larvae, 52/60 nerves; p < 0.001). All scale bars, 10 µm. |
|
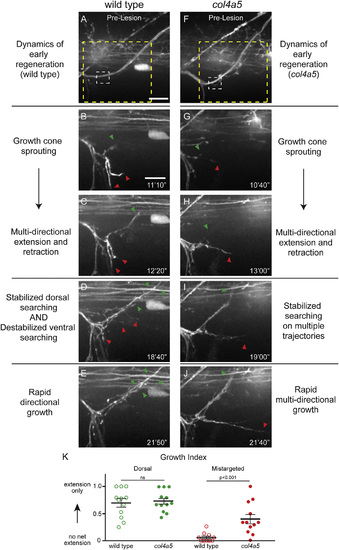
col4a5 Destabilizes Aberrant Growth Early in Regeneration (A) Wild-type dorsal nerve prior to nerve transection (white dashed box, transection site; yellow dashed box, region magnified in B–F; scale bar, 10 µm). (B and C) Wild-type axons sprout growth cones (B) and probe all regions of the injury gap (C). (D and E) Over time, axons destabilize searching on non-dorsal paths and stabilize searching on the dorsal path (D), leading to rapid directional growth (E; n = 8 larvae, 15/16 nerves). (F) col4a5 dorsal nerves develop indistinguishably from wild-type siblings (white dashed box, transection site; yellow dashed box, region magnified in H–L). (G and H) Regenerating col4a5 axons sprout growth cones (G) and search all regions of the injury gap (H). (I) Over time, axons stabilize searching on the dorsal path but fail to destabilize searching on non-dorsal paths. (J) This leads to invasion of non-dorsal regions of the myotome (n = 8 larvae, 17/25 nerves; p < 0.0001). Red arrowheads, ventral searching; green arrowhead, dorsal searching; scale bar, 10 µm. (K) The net proportion of extension and retraction movements was similar between wild-type and col4a5 fascicles on the dorsal path. In contrast, while wild-type fascicles extended and retracted with equal frequency, mistargeted col4a5 fascicles extended more frequently and stabilized to grow on aberrant trajectories. All error bars indicate ±SEM. |
|
Slit1a Is Upregulated with collagen4a5 after Nerve Transection (A) Schematic showing approximate region imaged for in situ hybridization (black dashed box, transection site). (B–D) col4a5 mRNA (B) and slit1a mRNA (C) are co-expressed (D) ventral to the spinal after nerve transection (n = 6 larvae, 27/30 nerves). White line, dorsal aspect of spinal cord; white dashed box, approximate transection site; scale bar, 10 µm. |
|
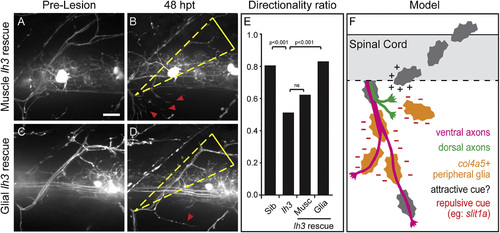
lh3 in Peripheral Glia Rescues lh3 Dorsal Axon Regeneration Defects (A–E) Pre-lesion (A) and 48 hpt (B) examples reveal that expression of lh3 in all muscles Tg(aActin:lh3mkate) fails to rescue lh3 axon regeneration. In contrast, pre-lesion (C) and 48 hpt (D) examples demonstrate that transgenic expression of lh3 in peripheral glia Tg(sox10:lh3mkate) significantly rescued these guidance defects (E). (F) After nerve transection, regenerating motor axons cross the injury gap to return to distal Schwann cells and reinnervate dorsal muscle targets. lh3 glycosylation of ECM components and the lh3 substrate col4a5 are required for targeting dorsal, but not ventral, motor axon regeneration. Col4a5 is upregulated in ventral and ventrolateral Schwann cells where it may act to present canonical guidance cues, such as slit1a, to destabilize regenerating axons. Dashed white boxes outline the nerve transection site; dashed yellow triangle, dorsal ROI; red arrowheads, aberrant regrowth; scale bar, 10 µm. |
|
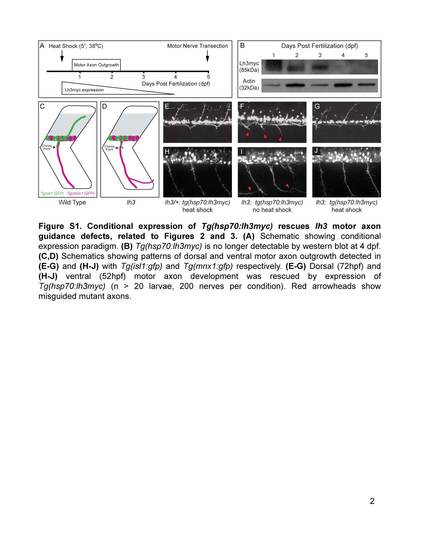
Conditional expression of Tg(hsp70:lh3myc) rescues lh3 motor axon guidance defects, related to Figures 2 and 3. (A) Schematic showing conditional expression paradigm. (B) Tg(hsp70:lh3myc) is no longer detectable by western blot at 4 dpf. (C,D) Schematics showing patterns of dorsal and ventral motor axon outgrowth detected in (E-G) and (H-J) with Tg(isl1:gfp) and Tg(mnx1:gfp) respectively. (E-G) Dorsal (72hpf) and (H-J) ventral (52hpf) motor axon development was rescued by expression of Tg(hsp70:lh3myc) (n > 20 larvae, 200 nerves per condition). Red arrowheads show misguided mutant axons. |
|
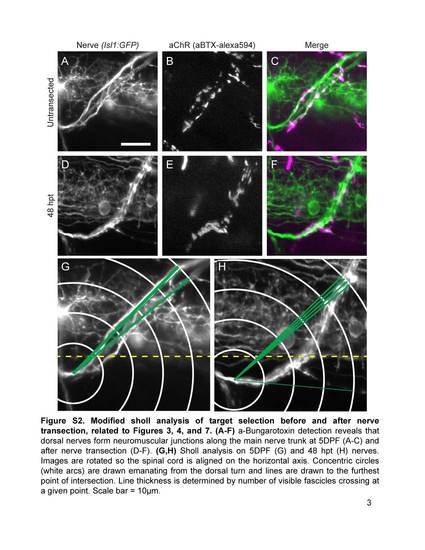
Modified sholl analysis of target selection before and after nerve transection, related to Figures 3, 4, and 7. (A-F) a-Bungarotoxin detection reveals that dorsal nerves form neuromuscular junctions along the main nerve trunk at 5DPF (A-C) and after nerve transection (O-F). (G,H) Sholl analysis on 5DPF (G) and 48 hpt (H) nerves. Images are rotated so the spinal cord is aligned on the horizontal axis. Concentric circles (white arcs) are drawn emanating from the dorsal turn and lines are drawn to the furthest point of intersection. Line thickness is determined by number of visible fascicles crossing at a given point. Scale bar = 10µm. |
|
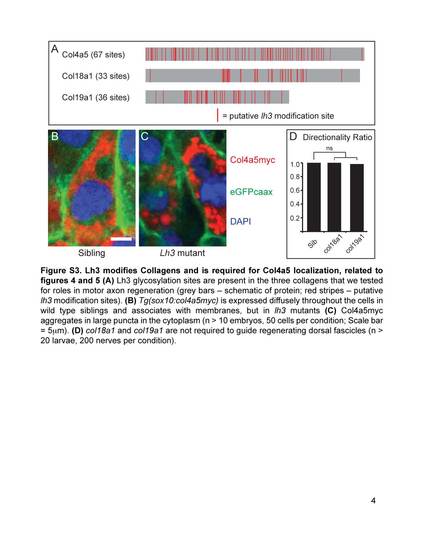
Lh3 modifies Collagens and is required for Col4a5 localization, related to figures 4 and 5 (A) Lh3 glycosylation sites are present in the three collagens that we tested for roles in motor axon regeneration (grey bars - schematic of protein; red stripes - putative lh3 modification sites). (B) Tg(sox10:col4a5myc) is expressed diffusely throughout the cells in wild type siblings and associates with membranes, but in lh3 mutants (C) Col4a5myc aggregates in large puncta in the cytoplasm (n > 10 embryos, 50 cells per condition; Scale bar = 5µm). (D) col18a1 and col19a1 are not required to guide regenerating dorsal fascicles (n > 20 larvae, 200 nerves per condition). |
|
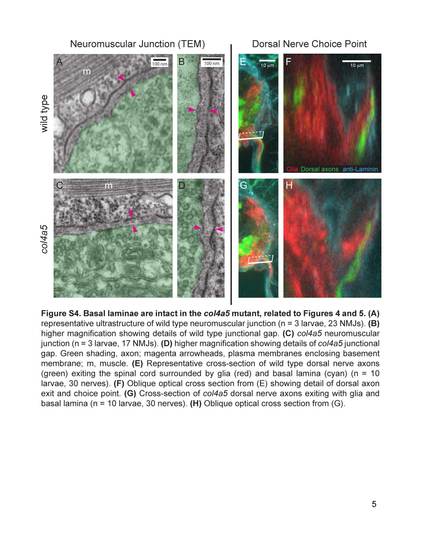
Basal laminae are intact in the col4a5 mutant, related to Figures 4 and 5. (A) representative ultrastructure of wild type neuromuscular junction (n = 3 larvae, 23 NMJs). (B) higher magnification showing details of wild type junctional gap. (C) col4a5 neuromuscular junction (n = 3 larvae, 17 NMJs). (D) higher magnification showing details of col4a5 junctional gap. Green shading, axon; magenta arrowheads, plasma membranes enclosing basement membrane; m, muscle. (E) Representative cross-section of wild type dorsal nerve axons (green) exiting the spinal cord surrounded by glia (red) and basal lamina (cyan) (n = 10 larvae, 30 nerves). (F) Oblique optical cross section from (E) showing detail of dorsal axon exit and choice point. (G) Cross-section of col4a5 dorsal nerve axons exiting with glia and basal lamina (n = 10 larvae, 30 nerves). (H) Oblique optical cross section from (G). |