- Title
-
Isthmus-to-midbrain transformation in the absence of midbrain-hindbrain organizer activity
- Authors
- Jászai, J., Reifers, F., Picker, A., Langenberg, T., and Brand, M.
- Source
- Full text @ Development
|
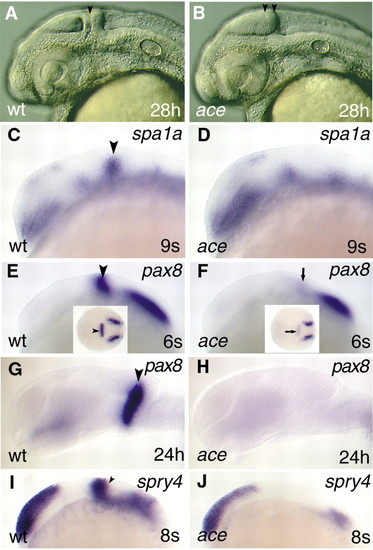
The molecular identity of the isthmic domain is not maintained in ace. All views are rostral to the left, and are lateral apsects, apart from the insets in E and F, which are dorsal views. (A,B) Captures of living embryos illustrating the special anatomical features (lack of isthmus and separate cerebellar anlage) of the ace mutants. The black arrowhead in panel A points to the isthmic constriction; the two black arrowheads in panel B mark the caudal tectal expansion. (C,D) Analysis of spa1a expression reveals the lack of the isthmic expression domain in the mutant embryos in comparison with wild type. The black arrowhead labels the MHB expressing spa1a in wild-type embryo. (E,F) Only a low level of pax8 expression can be detected at the MHB in the mutant embryos (arrows; F,F inset) in comparison with wild types (arrowheads; E,E inset). (G,H) Later on, expression of pax8 is abolished from the prospective MHB region in ace mutants. (I,J) In contrast to pax8, expression of spry4 is not initiated in the mutants. The arrowhead (I) marks the MHB expression domain of spry4 in the wild-type embryo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
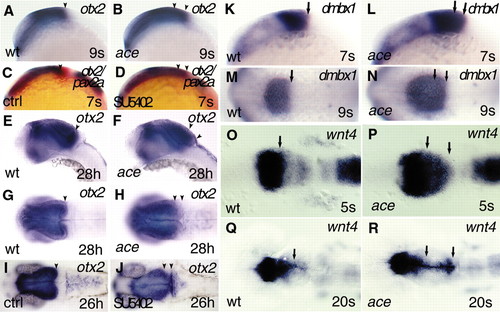
Caudal expansion of fore- and midbrain markers in ace mutants and SU5402 inhibitor-treated embryos. All views are rostral to the left; (A-F,K-L) lateral views; (G-J,M-R) dorsal views. (A-J) Analysis and comparison of otx2 expression by whole-mount in situ hybridization in wild-type, ace mutant and SU5402-treated embryos, at early somitogenesis (A-D), and at the pharyngula period (E-J). (A,B) In the mutant embryos, expression of otx2 is caudally shifted well before the period when the anatomical isthmic constriction should form. (C-F) In SU5402-treated embryos, expression of otx2 (blue) is markedly shifted, similar to ace mutants, whereas expression of pax2a (red) is reduced. The expanded otx2 territory is also characteristic for later developmental stages, both in ace mutants (E-H) and SU5402 inhibitor-treated embryos (I-J), as illustrated at 28 hpf and 26 hpf, respectively. The black arrowhead (A,C,E,G,I) points to the caudal expression limit of otx2 at the MHB of wild-type embryos. The two arrowheads (B,D,F,H,J) mark the caudally expanded territory expressing otx2 in ace mutant and SU5402-treated embryos. (K-N) As with otx2, expression of dmbx1 is markedly expanded at early somitogenesis in the mutant embryos, as illustrated at the 7- and 9-somite stage. The single arrow (K,M) labels the caudal limit of dmbx1 expression in the developing wild-type mesencephalic tectum. The two arrows (L,N) indicate the expanded expression of dmbx1 in ace mutant siblings. (O-R) Analysis of wnt4 expression reveals an early upregulation, and expansion of the fore-midbrain territory toward caudal coordinates of the mesencephalic alar plate in ace mutants. (O-P) The initially broad, expanded expression domain of wnt4 narrows to the dorsomedial parts of the alar plate, and is extended to the caudally enlarged tectal compartment at later somitogenesis stages, as illustrated at the 20-somite stage. The black arrows (O,Q) indicate the caudal limit of the enriched expression of wnt4 at the fore-midbrain region in wild-type embryos. The two arrows (P,R) show the markedly upregulated and expanded expression of wnt4 in ace mutants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
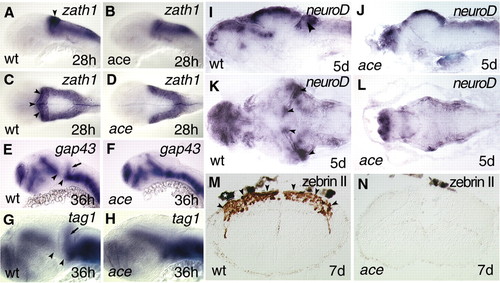
Lack of cerebellar development in ace mutants. (A-H) Whole-mount in situ hybridisations. (I-L) In situ hybridisation of sections. (M-N) Immunohistochemistry of sections. (A-L) rostral to left; (A,B,E,H) lateral views; (C,D) dorsal views; (I,J) lateral views of sagittal sections; (K,L) dorsal views of horizontal whole brain sections; and (M,N) transversal hindbrain sections. zath1 expression is not detectable in the upper rhombic lips of ace mutants (B,D) in comparison with wild-type (A,C) embryos. Arrowheads (A,C) point to the upper rhombic lips expressing zath1 in wild-type embryos. No migrating granule cell precursors can be detected by analysing gap43 (E,F) and tag1 (G,H) expression in ace mutants. Arrows (E,G) mark the migrating granule cell precursors in the developing cerebellar anlage. Note that the ventral mesencephalic expression domain of both gap43 and tag1 are fused to the hindbrain expression domain. Arrowheads mark the gap between the rostral and caudal expression domains of gap43 (E) and tag1 (G). (I-L) Expression analysis of neurod fails to detect a cerebellar compartment containing granule cell precursors in the mutants. Arrowheads (I,K) point to the cerebellar anlage expressing neurod mRNA. (M,N) Immunohistochemical visualization of zebrin II, the evolutionarily conserved marker of Purkinje cells, fails to detect any of these cells in ace mutants. Arrowheads (M) point to the cerebellar plate loaded with zebrin II-expressing cells (brown staining). The white asterisk above the hindbrain section (M) marks a pigment granule. |
|
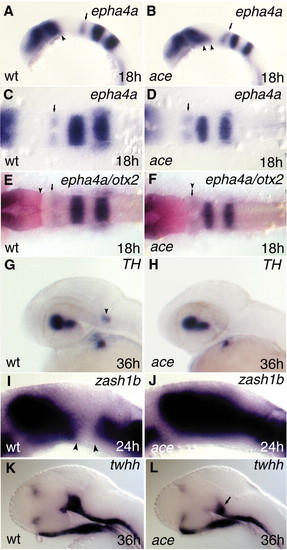
Analysing rhombomere 1 (r1) and the ventral mesencephalon in ace mutants. The rostralization stops at caudal r1 in ace mutants (A-H). The ventral mesencephalon displays altered gene expression profiles in ace mutants (A,B,I-L). All views are rostral to the left. A,B,G-L are lateral views; C-F are dorsal views. (A-D) Expression of epha4a, labeling the caudal part of r1, is still detectable in ace mutants. However, this domain in ace embryos (B) shows a distorted, slanted orientation in comparison with wild-type siblings (A), where it is approximately perpendicular to the axis of the hindbrain. Small arrows (A-D) point to the epha4a-expressing caudal r1 compartment. Note that the expression of epha4a at the fore-midbrain junction is expanded toward the ventral mesencephalon in ace mutants. Arrow (A) points to the caudal limit of epha4a expression at the fore-midbrain region. Two arrowheads (B) point to the expanded epha4a expression domain in the mutant embryo. (E,F) In the hindbrain, a new interface is detectable between the otx2 (red) and epha4a (purple) expression domains in ace embryos. The arrowhead (E) points to the caudal limit of otx2; the small arrow labels the rostral end of epha4a in the caudal r1 of wild-type embryos. The arrowhead above the small arrow (F) marks the new otx2/epha4a interface in the ace mutant. (G,H) The Locus ceruleus (LC; arrowhead in G) is missing from r1 in ace mutants. (I,J) In ace mutant embryos, the diencephalic expression domain of zash1b expands toward the mesencephalic tegmentum and fuses to the hindbrain expression domain (J). Arrowheads (I) mark the gap between the rostral and caudal expression domains of zash1b. (K,L) Expression of twhh is severely compromised in the ventral mesencephalic region of pharyngula-stage mutant embryos in comparison with wild-type embryos. The arrow (L) points to the reduced ventral mesencephalic twhh expression domain. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Comparison of cell proliferation and cell death patterns in wild-type embryos and ace mutants. (A,B,H,I,L,M) lateral views; (C-G,J,K,N,O) dorsal views. (A-G) Comparison of the cell proliferation characteristics of wild-type and mutant embryos using anti-phospho histone H3 (anti-PH3) whole-mount in situ hybridization staining. (A-D) No differences can be detected, during the mid-somitogenesis period, in the cell proliferation patterns of wild-type and ace mutant embryos after anti-PH3 immunostaining (brown reaction product). Arrowheads mark the range between the mesencephalon and rostral hindbrain under investigation. (E) Double immunostaining depicting the anatomical relationship of proliferation zones (anti-phospho histone; green) and axonal trajectories (anti-acetylated tubulin; red) of the mesencephalic tectal region during normal development, at 57 hpf. (F,G) Comparing the anti-PH3 staining pattern in 57 hpf wild-type and ace mutant larvae reveals a dramatic reduction of cell proliferation. The mutants lose the typical caudal tectal and cerebellar proliferation zones seen in wild-type larvae. The white arrowheads (F,G) delineate the caudal proliferation zones. (H-O) Analysis of cell death in wild-type and mutant embryos. (H,I) TUNEL staining (brown) fails to detect marked differences between wild-type and ace mutant embryos at early somitogenesis as demonstrated at the 8-somite stage. The arrowheads (H,I) label the region of interest for comparison. (J,K) At later stages of the segmentation period the amount of cell death is increasing in the mutant embryos in comparison with wild-type siblings, as revealed by Nomarski (DIC) optic. In the mutants, dead cells (small, round, excluded superficial structures) can be seen all over the midbrain and rostral hindbrain, but are more concentrated above the rostral hindbrain region. The arrowheads (K) indicate the area where a higher number of dead cells is visible. (L-O) Detecting apoptotic cell death (brown reaction product) at the 15-somite stage reveals an increased number of dead cells above the rostral hindbrain and r4 in ace mutant embryos (M,O; arrowheads). PHENOTYPE:
|
|
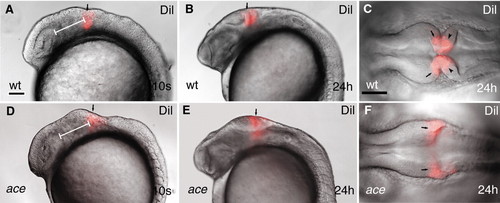
Dil lineage-tracing reveals fate alteration of MHB primordial cell in ace mutants. All views are rostral to the left. (A,B,D,E) Lateral views; (C,F) dorsal views. (A-F) Labeling (red) wild-type and ace mutant embryos at equivalent rostrocaudal positions along the neuraxis reveals that the labeled cells in the mutants are not retained in the MHB compartment. The labeled mutant cells always end up at the caudal enlargement of the tectum (E,F). Arrows (A,B,D,E) point to the Dil-labeled group of cells. Arrows (C,F) point to the mesencephalic side of the labeled compartment; arrowheads (C) point to the hindbrain side of the Dil-labeled cell population. The white bar (A,F) shows the distance between the caudal edge of the otic vesicle and the Dil injection. |
|
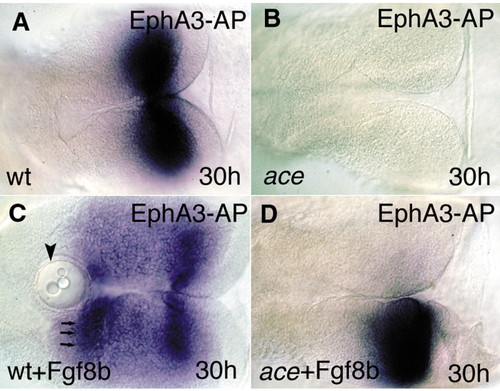
Fgf8-bead implantation restores the molecular and anatomical identity of the MHB territory. All views are rostral to the left, and are dorsal aspects. (A) The Epha3-AP fusion protein reveals the distribution of ephrin ligands (blue) in the mesencephalic tectum. (B) The fusion protein fails to detect the typical ephrin gradient expression in the mutant tecta. (C) After implanting Fgf8-coated beads into the wild-type diencephalon, a second, mirror gradient of ephrin A proteins can be detected. Arrowhead indicates the implanted beads; arrows indicate the second, ectopic ephrin A domain. (D) Unilateral implantation of the Fgf8-coated beads into ace embryos is able to restore the graded ephrin A expression on the operated side. |