- Title
-
Changes in retinoic acid signaling alter otic patterning
- Authors
- Hans, S., and Westerfield, M.
- Source
- Full text @ Development
|
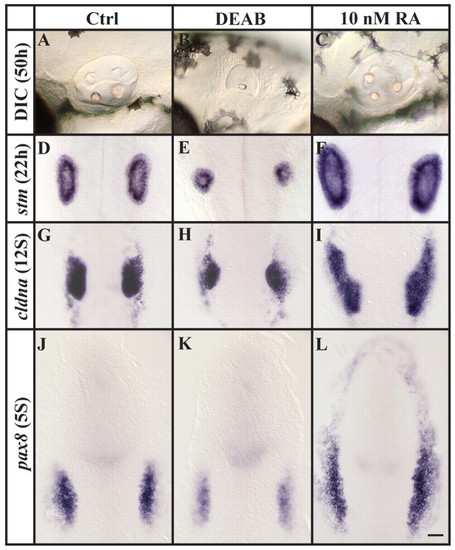
Loss and excess of RA signaling generate opposite phenotypes. (A-F) Compared to control embryos, otic vesicles are reduced or increased in size, respectively, after depletion of RA signaling (DEAB) or after the application of 10 nM RA, as assessed both by morphology at 50 hpf (A-C) or stm expression at 22 hpf (D-F). (G-L) Reduction or increase in the number of preotic cells is already evident at placodal (G-I, 12-somite stage) and preplacodal (J-L, 5-somite stage) stages after labeling with cldna (G-I) or pax8 (J-L). (A-C) Lateral views of live otic vesicles with anterior to the left and dorsal towards the top. (D-L) Dorsal views with anterior towards the top. Scale bar: 35 µm. EXPRESSION / LABELING:
|
|
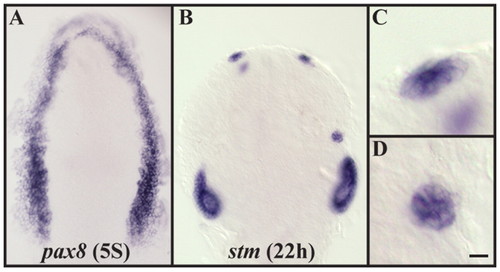
Application of 20 nM RA leads to widespread ectopic otic induction and to the formation of ectopic otic cells outside of the endogenous ear. (A) Application of 20 nM RA leads to ectopic pax8 expression encompassing the entire preplacodal domain (compare with Fig. 1J,L). (B-D) Embryos treated with 20 nM RA display ectopic patches of cells that express the otic marker stm and form enlarged vesicles. (C,D) Higher magnifications of B show the epithelial structure of these patches. Dorsal views with anterior towards the top. Scale bar: 35 µm for A; 50 µm for B; 10 µm for C,D. EXPRESSION / LABELING:
|
|
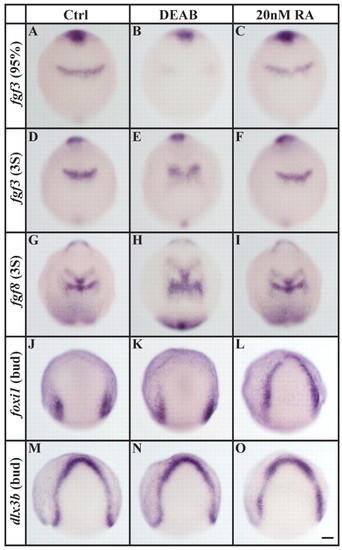
Loss and excess of RA signaling affect different tissues. (A-F) In comparison to controls (A,D) or to embryos treated with 20 nM RA (C,F), r4-specific fgf3 expression is delayed by the end of gastrulation in embryos depleted of RA signaling (DEAB, B) and remains reduced in an enlarged r4 primordium at the three-somite stage (E). (G-I) An enlarged r4-specific fgf8 expression domain is also present in embryos depleted of RA signaling (H) compared with control (G) or 20 nM RA-treated (I) embryos. (J-L) foxi1 expression is indistinguishable from control embryos (J) after RA-signaling depletion (K), whereas the application of 20 nM RA leads to ectopic expression within the entire preplacodal domain (L). (M-O) Expression of dlx3b in the preplacodal domain is unaffected by loss (N) or excess (O) of RA signaling. Dorsal views with anterior towards the top. Scale bar: 90 µm. EXPRESSION / LABELING:
|
|
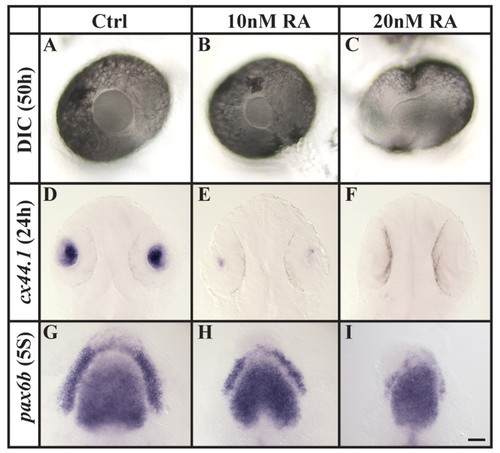
Increased RA signaling compromises lens specification. (A-F) Assessed both by morphology at 50 hpf (DIC microscopy, A-C) and cx44.1 expression at 24 hpf (D-F), the lens is reduced in size or completely lost after the application of 10 or 20 nM RA, compared with wild-type embryos. (G-I) Compromised lens specification is already evident at preplacodal stages (five-somite stage, 5S), as indicated by pax6b labeling. (A-C) Lateral views of live eyes with anterior to the left and dorsal towards the top. (D-I) Dorsal views with anterior towards the top. Scale bar: 30 µm for A-C; 50 µm for D-F; 35 µm for G-I. EXPRESSION / LABELING:
|
|
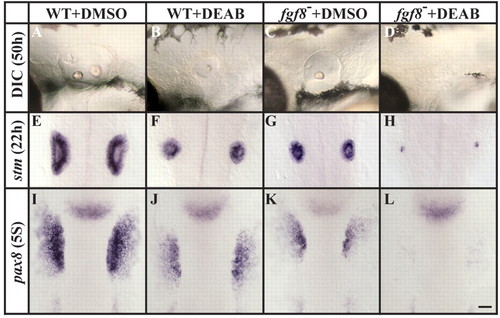
Fgf8 mediates otic induction in the absence of RA signaling. (A-D) Assessed by morphology at 50 hpf (DIC microscopy), otic vesicles are reduced after the depletion of RA (B) and in fgf8 mutants (C), and are completely lost in fgf8 mutants depleted of RA signaling (D), in comparison to controls (A). (E-H) Labeling with stm at 22 hpf reveals the presence of only residual otic cells in fgf8 mutants depleted of RA signaling (H) compared to fgf8 mutant (G), RA-depleted (F) or control (E) embryos, in which more otic cells are present. (I-L) Otic vesicle size reduction in fgf8 mutants depleted of RA signaling is evident as early as the preplacodal stages (five-somite stage, 5S), as detected by labeling with pax8. (A-D) Lateral views of live otic vesicles with anterior to the left and dorsal towards the top. (D-L) Dorsal views with anterior towards the top. Scale bar: 35 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
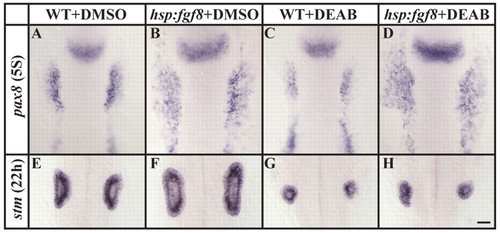
Misexpression of fgf8 at the end of gastrulation rescues otic induction in RA-depleted embryos, but the rescue is not maintained. (A,B) Preotic pax8 expression is expanded in transgenic hsp:fgf8 embryos (B) after misexpression of fgf8 at the end of gastrulation in comparison to non-transgenic embryos (A). (C,D) Reduced preotic pax8 expression in RA-depleted embryos (C) can be rescued by the misexpression of fgf8 at the end of gastrulation (D), as can heat-shocked transgenic hsp:fgf8 embryos with normal RA signaling (B). (E,F). Misexpression of fgf8 at late gastrulation stages (F) leads to the formation of much larger otic vesicles in comparison to their non-transgenic siblings (E). (G,H) Transgenic hsp:fgf8 embryos depleted of RA signaling (H) do not show an increase in otic cells but rather resemble non-transgenic RA-depleted siblings (G). Dorsal views with anterior towards the top. Scale bar: 35 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
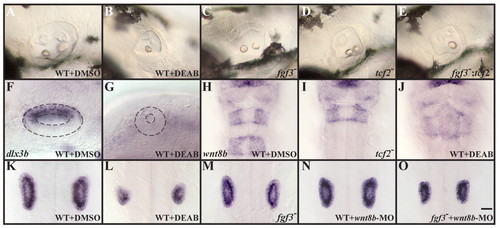
Rhombomere 5-specific Wnt8b function is required for the maintenance of otic fate. (A-D) Assessed by morphology, otic vesicles are reduced in fgf3 (C) and tcf2 (D) single mutants compared to wild-type embryos (A), but not as much as in RA-depleted embryos (B). (E) Loss of both fgf3 and tcf2 together results in otic vesicles that form only one otolith, as is observed in RA-depleted embryos (B). (F,G) Expression of dlx3b in the dorsal half of the otocyst at 22 hpf is lost in embryos depleted of RA signaling. (H-J) In control embryos at 22 hpf, wnt8b expression can be detected in the midbrain-hindbrain boundary, in r3 and in r5 (H), whereas tcf2 mutants (I) and RA-depleted embryos (J) show wnt8b in the midbrain-hindbrain boundary and in r3, but not in r5. (K-N) Inactivation of Wnt8b in wild-type embryos by morpholino injection (MO, N) leads to a reduced ear size (labeled with stm at 22 hpf), similar to the size observed in fgf3 mutants (M), compared to untreated wild type (K); however, the size reduction is not as severe as in embryos depleted of RA signaling (L). Loss of Wnt8b function in fgf3 mutants (O) produces reduced otic vesicles comparable to RA-depleted embryos (L). (A-G) Lateral views with anterior to the left and dorsal towards the top. (H-O) Dorsal views with anterior towards the top. Scale bar: 35 µm for A-E and H-O; 20 µm for F,G. EXPRESSION / LABELING:
PHENOTYPE:
|
|
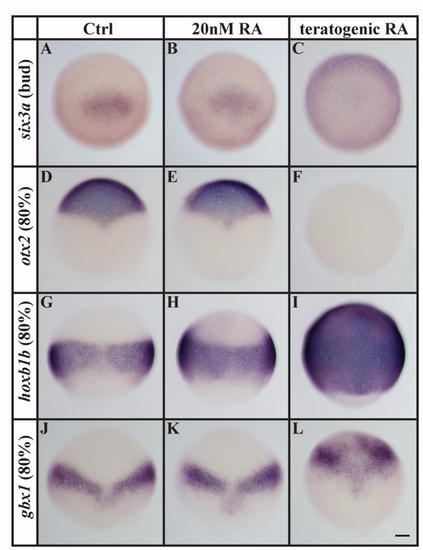
Teratogenic doses (1 μM), but not 20 nM, RA alter the expression of early markers of anterioposterior axis specification. (A-F) In comparison to controls, expression of the anterior neural plate markers six3a (A-C) and otx2 (D-F) is unaffected by 20 nM RA but is completely abolished in the presence of a teratogenic dose. (G-L) By contrast, expression of the posterior neural plate markers hoxb1b (G-I) and gbx1 (J-L) is greatly expanded in embryos treated with a teratogenic dose of RA, whereas embryos treated with 20 nM RA show only a mild or no anterior expansion in comparison to control embryos. Scale bar: 90 μm. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|