- Title
-
The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail
- Authors
- Schier, A.F., Neuhauss, S.C.F., Helde, K.A., Talbot, W.S., and Driever, W.
- Source
- Full text @ Development
|
Phenotype of oepm134 and oepz1 mutant embryos. (A-D) Comparison of wild-type (A), homozygous oepm134 (B), homozygous oepz1 (C) and transheterozygous oepm134/oepz1 (D) embryos at 27-28 hpf in a lateral view. Here and in all other figures anterior is to the left and dorsal is up, except where indicated otherwise. Note the general degeneration of the head region of homozygous oepz1 mutant embryos (arrow in C) but not homozygous oepm134 or transheterozygous oepm134/oepz1. (E-H) Comparison of the head region of wild-type (E), homozygous oepm134 (F), homozygous oepz1 (G) and transheterozygous oepm134/oepz1 (H) embryos at 27-30 hpf in a lateral view. Note the anterior-medial location of the single eye in oep mutants (arrow). + indicates the location of the midbrain-hindbrain boundary. (I,J) Ventral view of the head region of wild-type (I) and oepm134 mutant (J) embryos at 27-29 hpf. Arrows highlight the location of the two eyes in wild-type and the single median eye in oep mutant embryos. (K,L) Hatching gland formation over the yolk in wild-type (arrow in K) but not oepm134 mutants (L) at 38 hpf. Ventral view, anterior is up. (M-T) Notochord formation in wild-type (M,Q), oepm134 (N,P,R,T) and oepz1 (O,S) mutant embryos at 27-30 hpf. (M-P) Dorsal view of the anterior-most region of the notochord (outlined by dots). Note that the anterior extent of the notochord is normally found next to the otic vesicle (indicated by stars). In some oep mutants the anterior end of the notochord is located more posteriorly (P). (Q-T) Lateral view of the notochord in the trunk and tail region. Arrows outline the borders of the notochord. The floor plate and neurocoel lie directly dorsal to the notochord. Note the wavy and thinner appearance of the notochord in some oep mutants (T). |
|
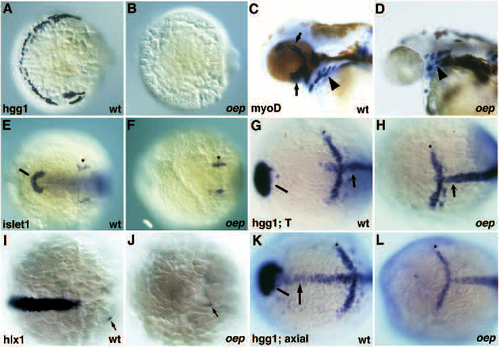
Defects in the formation of the prechordal plate and its derivatives in oep mutants. (A,B) Formation of hatching gland cells in wild-type (A) but not oep mutant (B) embryos at 26 hpf as judged from the expression of the hatching gland specific gene hgg1. Dorsal-anterior view, ventral is to the right. (C,D) Formation of eye muscles (arrows) in wild-type (C) but not oep mutant (D) embryos at 42 hpf as judged from expression of myoD. Arrowhead indicates the location of pharyngeal muscles in wild-type and oep mutant embryos. (E,F) Formation of hatching gland precursors (pillow) in wild-type (E, fine arrowhead) but not in oep mutant (F) embryos at the 6-somites stage as judged from islet1 expression. Stars indicate the normal formation of the trigeminal ganglion anlage. Dorsal view, anterior is to the left. (G,H) Formation of hatching gland precursors in wild-type (G, fine arrowhead) but not oep mutants at the end of gastrulation (bud stage) as judged from hgg1 expression. Note the normal anterior limit of notochord precursors anterior to the krox20 stripe in rhombomere 3 (star) as judged from the expression pattern of T/ntl (arrow). Dorsal view, anterior is to the left. (I,J) Expression of hlx1 in wild-type (I) and oep mutant embryos (J) at the tail bud stage. Arrows highlight the expression of hlx1 in the hindbrain. Dorsal view, anterior is to the left. (K,L) Expression of hgg1, axial and krox20 in wild-type (K) and oep mutant embryos (L) at 90% epiboly. Note that at this stage wild-type axial expression in the axial mesoderm still includes the prechordal plate (arrow) with the exception of the hatching gland precursors (fine arrowhead). Star indicates the location of krox20 expression in rhombomere 3. Dorsal view, anterior is to the left. EXPRESSION / LABELING:
|
|
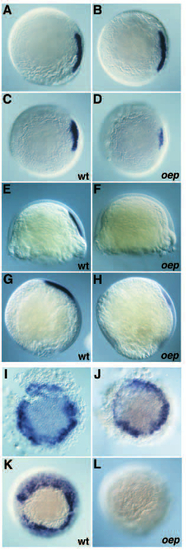
Expression of goosecoid is initiated but not maintained in oep mutants. (A-H) Gsc expression at 45% epiboly (A,B); 50% epiboly (C,D); after shield formation (E,F) and at 90% epiboly (G,H) in wild-type (C,E,G) and oep mutant embryos (D,F,H). (A-D) animal pole view, dorsal is to the right; (E-H) lateral view, dorsal is to the left, animal pole is up. Embryos in A,B could be either wild-type or mutant. Note the loss of gsc expression in oep mutants at the onset of involution. (I-L) Expression of gsc in Li-treated embryos; animal pole view. Embryos derived from a cross of oep/+ heterozygous fish were incubated in 0.3 M LiCl at the 256-512 cell stage as described by Stachel et al. (1993). (I,J) 45-50% epiboly; (K,L) 9.5 hpf. 28/28 (100%) Li-treated embryos derived from a cross of oep/+ heterozygous fish expressed gsc at 45-50% epiboly in the entire margin (I,J), but 18/57 (31% compared to an expected 25%) Li-treated embryos derived from a cross of oep/+ heterozygous lacked gsc expression at 9.5 hpf (L). 81/81 (100%) Li-treated embryos derived from a cross of wild-type fish expressed gsc at 9.5 hpf (K). EXPRESSION / LABELING:
|
|
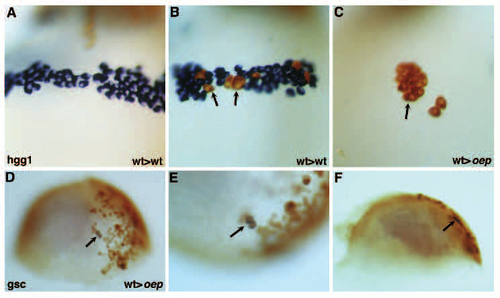
Cell-autonomous role of oep in prechordal plate formation. (A-C) Formation of hatching gland by wild-type donor cells transplanted into wild-type (A,B) or oep mutant (C) hosts; ventral view, anterior is up; 28 hpf. (A) No contribution of wild-type donor cells (brown) to the hatching gland (blue) of a wild-type host. (B) Contribution of wildtype donor cells (brown, arrows) to the hatching gland (blue) of a wild-type host. (C) Formation of hatching gland by wildtype donor cells (brown, arrow) in oep mutant host. No host hatching gland cells (blue) form. Hatching gland cells are large, are located over the yolk anterior to the head region, and have a characteristic granular appearance. 14/99 transplants of wild-type donor cells into oep mutant hosts resulted in the formation of hatching gland cells by wild-type donor cells but not mutant host cells. 0/44 transplants of oep mutant donor cells into wild-type hosts resulted in the formation of hatching gland cells by oep mutant cells. Transplantations were performed as described in Materials and Methods. (D-F) Expression of gsc by wild-type cells transplanted into oep mutant hosts at 70-80% epiboly following LiCl treatment. Donor cells expressing gsc (arrow) are brown (biotin-dextran) and blue (gsc mRNA). (D) Animal pole is up; (E) higher magnification; (F) side view of embryo in D and E. Note the location of gsc-expressing cells in the hypoblast. 11/41 transplants of wild-type donor cells into oep mutant hosts led to the formation of gsc-expressing wild-type donor cells but not gsc expressing oep mutant host cells. Donor and host embryos were treated with Li and transplantations were performed as described in Materials and Methods. |
|
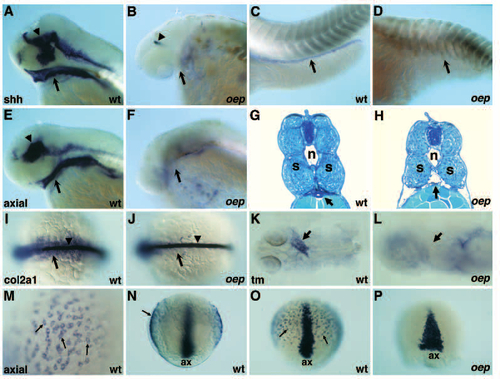
Endoderm formation is impaired in oep mutant embryos. (A-D) Expression of sonic hedgehog (shh) in wild-type (A,C) and oep mutant (B,D) embryos at 51 hpf. Expression of shh in the pharyngeal endoderm (arrow in A,B) and gut (arrow in C,D) is normal in wild-type but not in oep mutant embryos. Expression in the brain region of oep mutants (arrowhead in B) is strongly reduced as compared to wild-type (arrowhead in A). (E,F) Expression of axial in wild-type (E) and oep mutant (F) embryos at 51 hpf. Expression of axial in the pharyngeal endoderm (arrow in E,F) is normal in wild-type but not in oep mutant embryos. Expression in the brain of oep mutants is also severely affected as compared to wild-type (arrowhead in E). Sagittal cross-section (5 µm) of the trunk region of wild-type (G) and oep mutant (H) embryo at 53 hpf. Arrow indicates the location of the gut in wild-type (G) embryo. Note the lack of tissue and the gaping hole in this region of oep mutants (arrow in H). n, notochord; s, somites. (I,J) Expression of the type II collagen gene col2a1 in wild-type (I) and oep mutant (J) embryos at the 12-somites stage. Note the normal expression in the notochord (arrowhead), but severe reduction in the endoderm (arrow) of oep mutants (J). Dorsal view, anterior is to the left. (K,L) Expression of a-tropomyosin (tm) in the heart region (arrow) of wild-type (K) and oep mutant (L) embryos at 28 hpf. Note the severe reduction of tm in oep mutants. (M-P) Expression of axial in wild-type (M-O) and oep mutant embryos (P) at 80% epiboly. (M) High magnification view of axial expressing cells (arrows) located in the hypoblast. (N) Optical cross section reveals direct juxtaposition of axial-expressing cells to yolk (arrow), ax, axial expression in axial mesoderm. (O) Axial expression in axial mesoderm (ax) and presumptive endoderm (arrows) in wild-type embryos. (P) Loss of axial expression in the presumptive endoderm of oep mutants; ax, axial expression in the axial mesoderm of oep mutant embryos. Axial expression is laterally expanded as a result of reduced convergence and extension in oep mutants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
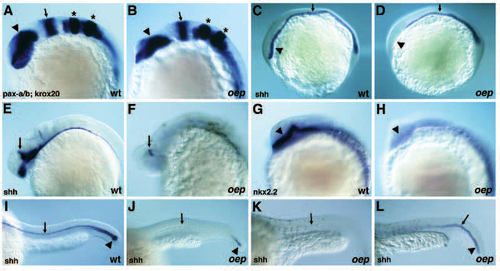
Neuroectodermal phenotype of oep mutant embryos. (A,B) Expression of pax-a (arrowhead) in the forebrain, pax-b (arrow) at the midbrainhindbrain boundary) and krox-20 (stars) in rhombomeres 3 and 5 in wild-type (A) and oep mutant (B) embryos at the 10-somites stage. Note the apparently normal anterior-posterior organization of expression patterns. (C,D) Expression of shh in wild-type (C) and oep mutant (D) embryos at the 1-somite stage. Note the absence of shh expression in the head region (arrowhead) of oep mutants. shh is expressed in the notochord, but expression in the floor plate is reduced or absent (arrow) in oep mutant embryos. (E,F) Expression of shh in the head region of wild-type (E) and oep mutant (F) embryos at 26 hpf. shh is only found in the dorsal-most region next to the epiphysis in oep mutants (arrow). (G,H) Expression of nk2.2 in the head region of wild-type (G) and oep mutant (H) embryos at the 18-somites stage. Arrowhead highlights expression in the ventral diencephalon. shh and nk2.2 are expressed in adjacent domains, shh being the more ventral marker. (I-L) Expression of shh in the tail and trunk region of wild-type (I) and oep mutant (J-L) embryos at 26-28 hpf. Note that the floor plate (arrow) is lost (J) or reduced (K,L) in oep mutants. Some oep mutant embryos (L) show a more persistent expression of shh in the notochord (arrowhead). EXPRESSION / LABELING:
|
|
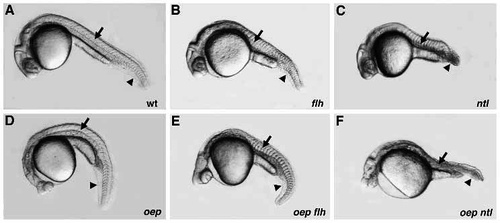
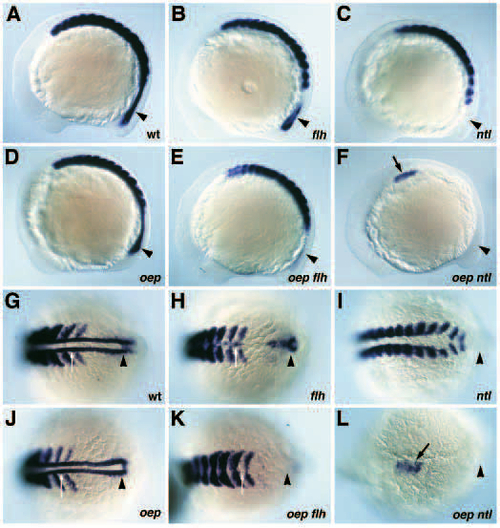
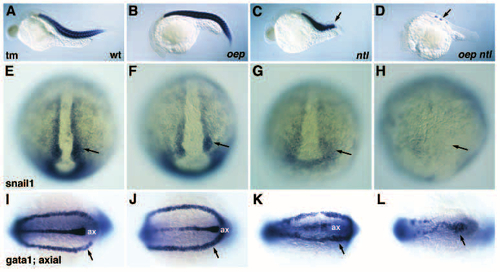
Phenotype of oep flh and oep ntl double mutants. Comparison of wild-type (A), and floating head (flh; B), no tail (ntl; C), one-eyed pinhead (oep; D), one-eyed pinhead; floating head double (oep flh; E), and one-eyed pinhead; no tail double (oep ntl; F) mutant embryos at 28 hpf. Arrow indicates the notochord, arrowhead indicates posterior region. Note the mainly additive features of oep flh double mutants as compared to the severe defects in oep ntl double mutants. PHENOTYPE:
|
|
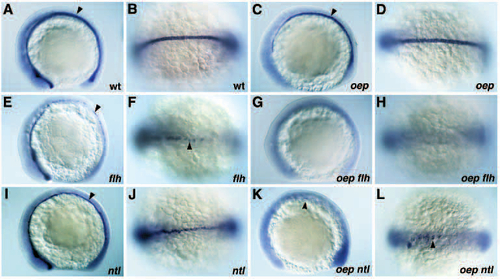
Midline defects in oep flh and oep ntl double mutants. (A-L) Expression of shh in wild-type (A,B), oep mutant (C,D), flh mutant (E,F), ntl mutant (I,J), oep flh double mutant (G,H) and oep ntl double mutant (K,L) embryos at the 11-12 somites stage. (A,C,E,G,I,K) lateral view; (B,D,F,H,J,L) dorsal view of anterior trunk region. (A,B) Expression of shh in ventral neuroectoderm including ventral diencephalon and floor plate (arrowhead), and notochord in wild-type embryos. (C,D) Expression of shh in notochord and few floor plate cells (arrowhead) of oep mutants. (E,F) Expression of shh in ventral neuroectoderm, anterior floor plate and scattered posterior floor plate cells (arrowhead) in flh mutants. (G,H) Absence of shh expression in oep flh double mutants. (I,J) Expression of shh in the midline in presumptive floor plate cells (arrowhead) of ntl mutants. (K,L) Expression of shh in scattered cells in the anterior trunk (arrowhead) of oep ntl double mutants. EXPRESSION / LABELING:
|
|
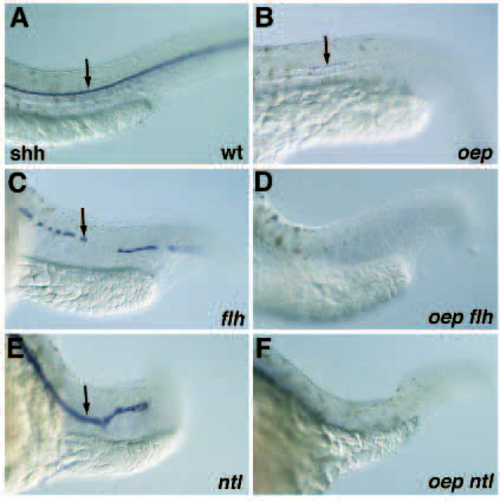
Floor plate formation in oep flh and oep ntl double mutants. (A-F) Floor plate formation (arrow) detected by the expression of shh in the trunk/tail region of wild-type (A), oep (B), flh (C), oep flh (D), ntl (E) and oep ntl (F) mutant embryos at 28 hpf. Note that the floor plate appears thicker in ntl mutant embryos as compared to wild-type embryos. EXPRESSION / LABELING:
|
|
Expression of myoD in oep flh and oep ntl double mutants. (A-F) Lateral view of myoD expression in wild-type (A), flh (B), ntl (C), oep (D), oep flh (E) and oep ntl (F) mutant embryos at the 11-12 somites stage. (G-L) Dorsal view of myoD expression in wild-type (G), flh (H), ntl (I), oep (J), oep flh (K), and oep ntl (L) mutant embryos. G-K display the posterior expression domain of myoD, (L) displays the anterior most and only expression domain of myoD in oep ntl double mutants. Adaxial cells located between the last two presumptive somites expressing myoD are indicated by a white arrow. Note that this cell population is fused in flh mutant embryos (H) and that myoD expression at this position is absent in ntl mutant and oep flh double mutant embryos. The posterior-most expression domain of myoD (arrowhead) is also drastically reduced in oep flh double mutants and absent in ntl mutants. Black arrow in F,L indicates the formation of a cluster of myoD expressing cells in the anterior trunk of oep ntl double mutants. EXPRESSION / LABELING:
|
|
Expression of mesodermal markers in oep ntl double mutants. (A-D) Expression of a- tropomyosin (tm) in wild-type (A), oep mutant (B), ntl mutant (C) and oep ntl double mutant (D) embryos at 26 hpf. Wild-type and oep mutant embryos have 30-32 somites at this stage, whereas ntl mutants have 15-17 somites (arrow). Note the dramatic deficit in tm expression in oep ntl double mutants (arrow in D). (E-H) Expression of snail 1 in wild-type (E), oep mutant (F), ntl mutant (G), and oep ntl double mutant (H) embryos at the bud stage. Note (arrows) the mild reduction of snail1 expression in ntl mutant embryos (G), and the severe deficit of snail1 expression in oep ntl double mutants (H). (I-L) Expression of gata1 and axial in wild-type (I), oep mutant (J), ntl mutant (K), and oep ntl double mutant (L) embryos at the 11-somites stage. Note the severe reduction of gata1 expressing cells (arrow) in oep ntl double mutant embryos (L); ax, axial expression in the midline. Assignment of gata1 (arrow) and axial (ax) expression domains is based on the analysis of embryos stained with either probe alone (data not shown). |