- Title
-
The PDZ domain protein Mcc is a novel effector of non-canonical Wnt signaling during convergence and extension in zebrafish
- Authors
- Young, T., Poobalan, Y., Tan, E.K., Tao, S., Ong, S., Wehner, P., Schwenty-Lara, J., Lim, C.Y., Sadasivam, A., Lovatt, M., Wang, S.T., Ali, Y., Borchers, A., Sampath, K., Dunn, N.R.
- Source
- Full text @ Development
|
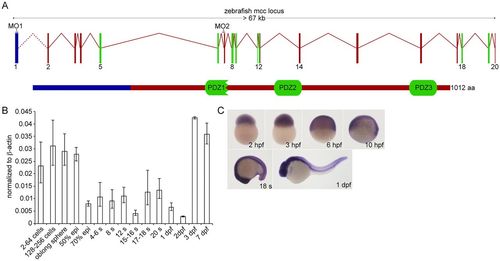
Zebrafish Mcc structure and embryonic expression. (A) Schematic of the zebrafish mcc genomic locus. The exact distance between exons 1 and 2 is unknown (dotted line). The 1012 amino acid (aa) Mcc protein (lower schematic) contains three PDZ domains. (B) Q-PCR analysis of mcc expression in the zebrafish embryo at maternal (2-256 cells) and zygotic (oblong sphere and beyond) stages. Data are normalized to β-actin. Error bars indicate s.e.m. epi, epiboly; s, somite. (C) mcc expression from 2hpf to 1dpf assessed by WISH. |
|
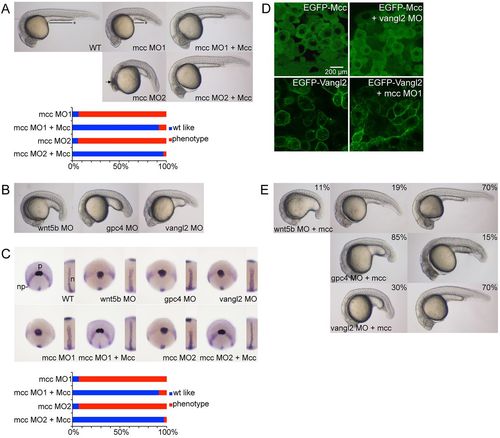
mcc acts downstream of wnt5b and vangl2 to regulate convergence and extension. (A) Injection of ATG (MO1) or splice junction (MO2) MOs targeting mcc yields zebrafish embryos with a shorter and ventrally curved anteroposterior axis with tightly packed somites at 1dpf. MO1 and MO2 positions are indicated in Fig. 1A. The black line with asterisk emphasizes the shortened yolk extension, and curved dotted line highlights the smaller eyes and reduced head development in mcc morphants compared with wild type (WT). The arrow indicates the region of anterior cell death. These defects are rescued by co-injection of mouse Mcc mRNA (see also supplementary material Fig. S2A). (B) Representative wnt5b, gpc4 and vangl2 morphants at 1dpf. (C) Triple ntl, hgg1 and dlx3 WISH at 10hpf. In wild-type embryos, hgg1 marks the polster (p) that lies on the anterior neural plate (np) boundary expressing dlx3. ntl1 identifies the thin midline notochord (inset). In morphants, the neural plate is wider mediolaterally, the notochord is thickened and shortened, and the polster is abnormally elongated posteriorly. Co-injection of Mcc mRNA and either mcc MO1 or MO2 yields hgg1/dlx3/ntl expression patterns that are indistinguishable from those of wild type (see also supplementary material Fig. S2B). (D) Lateral images of shield stage embryos injected as indicated. EGFP-Mcc is exclusively cytoplasmic in both wild-type and vangl2 morphant embryos. EGFP-Vangl2 is principally membrane localized in wild-type embryos, but is also visible in cytoplasmic puncta. EGFP-Vangl2 distribution is unaltered in mcc morphants. The N-terminal GFP-Xenopus laevis Mcc fusion (supplementary material Table S3) efficiently rescues zebrafish mcc morphants (data not shown). (E) Zebrafish mcc mRNA rescues loss of either wnt5b or vangl2 but not gpc4 at 1dpf. Combining mcc mRNA and wnt5b MO results in three phenotypic classes, as evidenced by variable yolk (and body axis) extension and head position. (A-E) MO and mRNA concentrations are provided in supplementary material Table S3. (A,C,E) Phenotypic distributions are indicated as percentages, with scored embryo counts listed in supplementary material Table S4. |
|
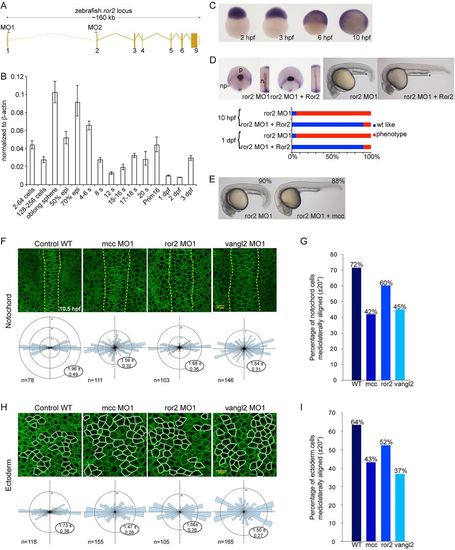
ror2 is required for convergence and extension in zebrafish and acts upstream of mcc. (A) Schematic of the ror2 locus, with the position of splice junction MOs (MO1 and MO2) indicated. (B) Q-PCR analysis of ror2 expression in developing zebrafish. Maternal ror2 transcripts are detected from 2-256 cells, with dynamic expression over the next 3days. Data are normalized to β-actin. Error bars indicate s.e.m. epi, epiboly; s, somite. (C) ror2 WISH at the indicated stages. (D) Injection of ror2 MO1 results in CE defects at 10hpf, as assessed by ntl, dlx3 and hgg1 triple in situ hybridization; these defects are robustly rescued at 10hpf and 1dpf by mouse Ror2 mRNA. The solid black line with asterisk emphasizes the shortened yolk extension at 1dpf, and the curved dotted line highlights the smaller eyes and head in ror2 morphants compared with wild type. np, neural plate; p, polster; n, notochord. (E) Co-injection of ror2 MO1 and zebrafish mcc mRNA results in ostensibly normal embryos at 1dpf. (F,H) Membrane GFP-labeled notochord (F) and dorsal ectoderm (H) cells of wild type and mcc, ror2 or vangl2 morphants as indicated at 90-95% epiboly (9.5-10hpf). Dorsal view with anterior upwards. The notochord boundary in F is marked by vertical dashed yellow lines. Note the extra columns of cells in the morphant notochords. Dorsal ectoderm cells selected for morphometric analysis are outlined in H in white. The number of cells analyzed (n) and length-to-width (LWR) ratios (expressed as mean±s.e.m.) are indicated in the Rose diagrams, which depict cell orientation relative to the embryonic anterior-posterior axis (vertical dashed line). (G,I) The percentage of mediolaterally aligned cells with a longitudinal axis oriented ±20° with respect to the embryonic mediolateral axis (horizontal dashed line in the Rose diagrams). (D,E,F,H) MO and mRNA concentrations are provided in supplementary material Table S3, with scored embryo counts listed in supplementary material Table S4. EXPRESSION / LABELING:
PHENOTYPE:
|
|
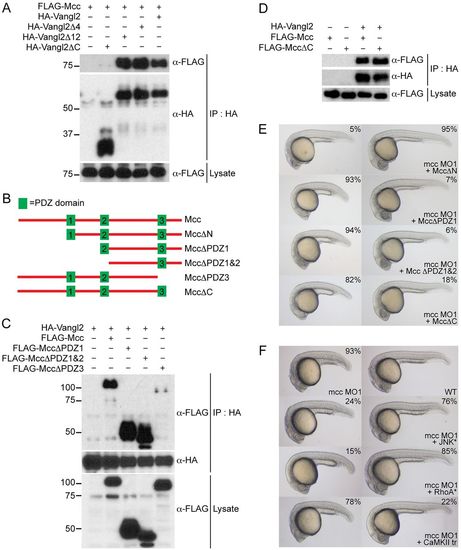
Mcc binds Vangl2 and acts upstream of RhoA and JNK. (A) Co-immunoprecipitation of FLAG-Mcc and HA-Vangl2 in HEK293 cells. Vangl2Δ4 (ΔETSV), Vangl2Δ12 and Vangl2ΔC correspond to increasing deletions of the Vangl2 cytoplasmic tail. Only Vangl2ΔC fails to precipitate FLAG-Mcc. Marker sizes (kDa) are indicated alongside. (B) Structure of wild-type mouse Mcc (top, not to scale) with three PDZ domains (green boxes) and assorted Mcc deletion constructs used for (C,D) immunoprecipitation and (E) zebrafish co-injection experiments. (C) HA-Vangl2 efficiently precipitates all Mcc deletion constructs except PDZΔ3, which lacks PDZ3 and the Mcc C-terminus. (D) HA-Vangl2 also efficiently binds MccΔC, which retains all three PDZ domains but lacks the extreme C-terminal ETSL type I PDZ interaction motif. (E) MccΔN mRNA rescues mcc morphants, whereas MccΔPDZ1, MccΔPDZ1&2 and MccΔC fail to complement loss of mcc, resulting in characteristic CE phenotypes. (F) Activated JNK* (MKK7B2Jnk1a1) and RhoA* (RhoA-G14V) but not CaMKII-tr rescue mcc morphants. The first row shows a representative mcc MO1 morphant (left) and an uninjected wild-type embryo (right) for reference. All embryos are at 1dpf. (E,F) MO and mRNA concentrations are provided in supplementary material Table S3. Phenotypic distributions are indicated as percentages, with scored embryo counts listed in supplementary material Table S4. |
|
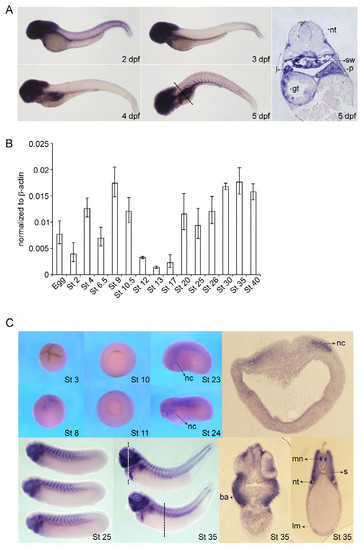
Later expression of zebrafish mcc and cloning and expression of Xenopus laevis Mcc. (A) Zebrafish mcc expression from 2 to 5 days post fertilization (dpf) by whole-mount in situ hybridization (WISH). mcc transcript levels in the somites decrease and then increase, but persist in head structures and also emerge in the developing digestive system and swim bladder. Section of 5 dpf embryo (far right panel); plane of section indicated by dashed line. Abbreviations: gt, gut tube; l, liver; p, pancreas; sw, swim bladder; nt, neural tube. (B) Expression analysis of Xenopus laevis Mcc by QPCR. Mcc transcripts are detected both maternally (up to stage (St) 8) and zygotically (St 10 and beyond). (C) Xenopus Mcc expression from St 3 to 35 by WISH and vibratome sections of St 18 and 35 embryos at the indicated planes (white and black dashed lines). St 3 (4-cell stage), animal view; St 8, lateral view; St 10 and 11, vegetal view; St 23 and 24, lateral view. Abbreviations: nc, neural crest; ba, branchial arches; mn, motor neurons; nt, nephric tubules; s, somite; lm, lateral mesoderm. |
|
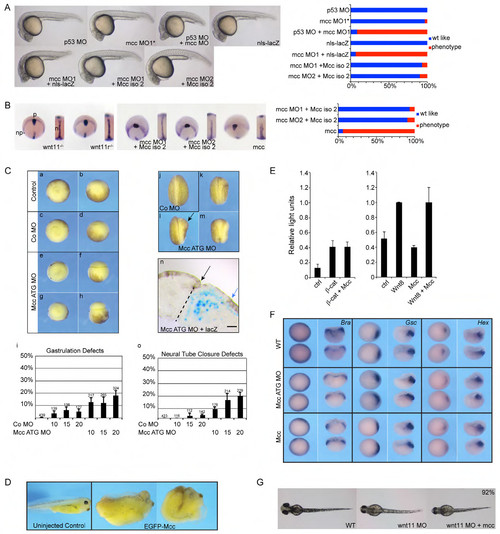
Supporting information for Figure 2. (A) Series of control mRNA and morpholino injections at 1 day post fertilization (dpf): p53 MO alone; mcc mismatch control morpholino (MO1*); co-injection of p53 MO and mcc MO1, which attenuates the anterior cell death sometimes observed with either mcc MO1 or MO2 (Fig. 2A); overexpression of nls-lacZ mRNA; and co-injection of mcc MO1 and nls-lacZ mRNA. Injection of the shorter mouse Mcc isoform 2 mRNA, like isoform 1 (Fig. 2A), efficiently rescues both mcc MO1 and MO2. (B) wnt11/slb and wnt11-related (wntllr) homozygous mutant embryos (Heisenberg et al., 2000; Matsui et al., 2005) at 10 hpf show characteristic convergence and extension defects: the polster (hgg1) lags behind the wider neural plate (dlx3) and the midline notochord (ntl1) is thickened. These phenotypes are seen in mcc MO1 or MO2-injected embryos and are rescued equally as efficiently by mouse Mcc isoform 2 mRNA as isoform 1 (Fig. 2A). Overexpression of zebrafish mcc results in similar CE defects as mcc MO1 or MO2 injection (Fig. 2A). Abbreviations: np, neural plate; p, polster; n, notochord. (C) Mcc loss of function in Xenopus leads to an increase of embryos with gastrulation and neural tube closure defects. Embryos were injected at the 2-cell stage with 10, 15, or 20 ng control or Mcc ATG MO. Gastrulation defects were analyzed at stage 11 (a-i) and neurulation defects (j-o) at stage 19. Vegetal (a,c,e,g) or lateral (b,d,f,h) views of gastrula stage embryos are shown. Neurula stage embryos are shown from a posterior view, the injected site is on the right (j-m). (a,b) Uninjected control embryo. (c,d) Embryo injected with 20 ng control MO (Co MO) showing normal gastrulation. (e-h) Embryos injected with 20 ng Mcc ATG MO showing defective blastopore closure (e,f) and exogastrulation (g,h). (i) Graph summarizing the percentage of gastrulation defects in three independent experiments. MO concentrations (10, 15 or 20) are in ng. Standard error of the mean and number of injected embryos are indicated for each column. The same embryos were analyzed for neural tube closure defects at stage 19 (j-m,o). (j) Embryo injected with 20 ng control MO shows normal neural tube closure. (k,l,m) Embryos injected with 20 ng Mcc ATG MO show defective neural tube closure on the injected site (right). (n) Transverse section through the neural tube of an embryo injected with 20 ng Mcc ATG MO together with lacZ RNA (blue staining, indicates the injected side; dashed line indicates the midline). Black arrow marks the hinge point and the rolled up neural tube of the control site. The blue arrow marks the hinge point area of the injected side. Note the distance between the blue arrow and the midline suggesting defects in cell intercalation on the injected site. Scale bar = 50 μm. (o) Graph summarizing the percentage of neural tube closure defects of the same embryos as shown in (i). (D) Mcc overexpression (2ng of EGFP-Mcc mRNA) in Xenopus results in foreshortening and buckling of the anteroposterior axis—two classic CE phenotypes (St 38). (E) Activation of the canonical, β-catenin-dependent Siamois luciferase reporter (Brannon et al., 1997) is not affected by Mcc overexpression in injected Xenopus embryos. See Materials and Methods for additional details about luciferase reporter constructs and DNA/mRNA concentrations. (F) Early gastrulation and germ layer specification are unperturbed in Xenopus Mcc morphants. Whole-mount in situ hybridization for Brachyury (Bra, pan-mesodermal), Gsc (dorsal organizer) and Hex (nascent endoderm) expression at St 10.5. Alternating animal pole and hemisected views are shown for each riboprobe with dorsal to the right. (G) Zebrafish embryos at 52 hpf. Wild-type (WT) uninjected, wnt11 MO injected, and co-injection of wnt11 MO with zebrafish mcc mRNA. wnt11 morphants typically have eyes that are too close together or fused (Heisenberg et al., 2000)—a phenotype that is not rescued upon mcc overexpression. (A-E) Morpholino and mRNA concentrations are provided in Supp. Table 3. Phenotypic distributions are indicated as percentages, with scored embryo counts listed in Supp. Table 4. |
|
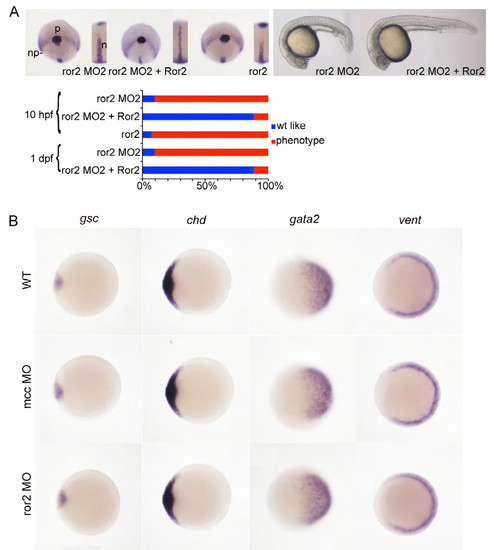
Characterization of a second ror2 splice junction morpholino. (A) ror2 MO2 phenotypes at 10 and 24 hours post fertilization (hpf) are superimposable with ror2 MO1-injected embryos (Fig. 3D). Whole-mount in situ hybridization (WISH) for hgg1, dlx3 and ntl transcripts identifies the polster (p), neural plate (np) boundary and notochord (n, inset), respectively, at 10 hpf. Co-injection of mouse Ror2 mRNA and ror2 MO2 rescues CE defects. Overexpression of zebrafish ror2, like mcc (Supp. Fig. 2C), also results in abnormal CE at 10 hpf. Morpholino and mRNA concentrations are provided in Supp. Table 3. Phenotypic distributions are indicated as percentages, with scored embryo counts listed in Supp. Table 4. (B) WISH for zebrafish dorsal (gsc and chd) and ventral (gata2 and vent) marker genes at shield stage. Both mcc and ror2 morphants display expression patterns identical to the wild type, indicating that dorsal-ventral patterning is normal in morphant embryos. Animal pole views with dorsal to the left. |
|
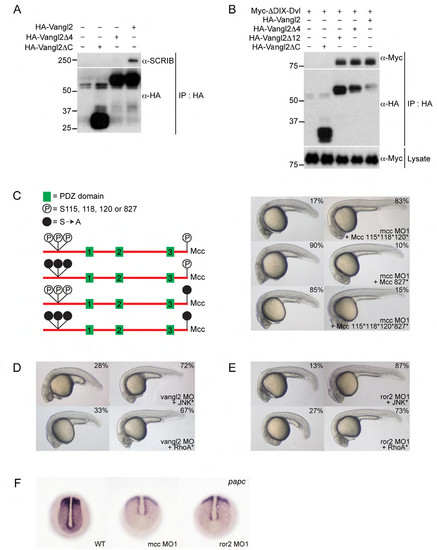
Supporting information for Figure 4. (A) HA-Vangl2 efficiently precipitates endogenous SCRIB in HEK293 cells. Deleting the extreme C-terminal ETSV type I PDZ interaction motif (indicated as Vangl2Δ4) disrupts this interaction. (B) HA-Vangl2, HA-Vangl2Δ4 and HA-Vangl2Δ12 efficiently bind ΔDIX-Dvl—a Xenopus Dishevelled N-terminal truncation that retains its single, central PDZ domain. Deleting the Vangl2 cytoplasmic tail (Vangl2ΔC) eliminates this interaction. These results indicate that Vangl2 binds Dvl via an unconventional PDZ interaction motif within its cytoplasmic tail. (C) Structure of mouse Mcc isoform 2 (not to scale) and location of previously mapped N-terminal (positions 115, 118 and 120) and extreme C-terminal (position 827 (or -1)) serine phosphorylation sites (Pangon et al., 2010; Pangon et al., 2012). Solid black circles indicate mutation of serine residues to non-phosphorylable alanine (S[right arrow]A). Co-injection of 115*, 118* and 120* mutant Mcc mRNA complements mcc loss equally as effectively as wild-type Mcc mRNA (Fig. 2A; Supp. Fig. 2A). In contrast, the C-terminal S827A mutation fails to rescue. (D,E) Activated JNK* (MKK7B2Jnk1a1) and RhoA* (RhoA-G14V) rescue vangl2 and ror2 morphants. (F) Whole-mount in situ hybridization for paraxial protocadherin (papc) expression in the posterior paraxial mesoderm of wild-type and mcc and ror2 morphant embryos at the bud/1-somite stage. Morphants show lower and more diffuse papc expression. (C-E) Morpholino and mRNA concentrations are provided in Supp. Table 3. Phenotypic distributions are indicated as percentages, with scored embryo counts listed in Supp. Table 4. |
|
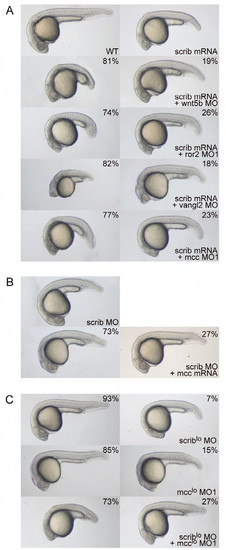
Mcc and Scribble are not in a simple genetic pathway. (A) Overexpression of zebrafish scrib mRNA yields minor CE defects—shortened body axis, displaced head, slight ventral flexure—consistent with published reports (Li et al., 2013; Wada et al., 2005). Co-injection of scrib mRNA with either wnt5b, ror2 or mcc morpholinos results in highly penetrant CE phenotypes that strongly resemble those of the individual morphants (Figs. 2A,B and 3D,E; Supp. Figs. 2A and 3A), indicating no rescue by scrib mRNA. Co-injection of vangl2 MO and scrib mRNA, however, yields a strong genetic interaction (enhanced CE phenotype) that has been previously documented (Wada et al., 2005). Note the further diminished anterior development, slight yolk extension and significantly shortened overall body axis length. (B) The weak scrib MO phenotype (Li et al., 2013; Wada et al., 2005) is not rescued by co-injection of mcc mRNA. (C) scrib and mcc MO concentrations that normally do not give rise to CE phenotypes are denoted with an asterisk (MO* and MO1*). 93% and 85% of scrib MO* and mcc MO1* singly injected embryos, respectively, display wild-type morphology. In contrast, co-injection of scrib MO* and mcc MO1* unmasks a strong “synthetic” interaction, as the doubly morphant embryos show greatly diminished anterior development (a hammerhead phenotype) and foreshortened body axis. This result provides strong evidence that Mcc and Scrib act in parallel signaling pathways that converge on the regulation of similar cellular processes. (AC) Morpholino and mRNA concentrations are provided in Supp. Table 3. Phenotypic distributions are indicated as percentages, with scored embryo counts listed in Supp. Table 4. |