- Title
-
Prdm1a directly activates foxd3 and tfap2a during zebrafish neural crest specification
- Authors
- Powell, D.R., Hernandez-Lagunas, L., Lamonica, K., and Artinger, K.B.
- Source
- Full text @ Development
|
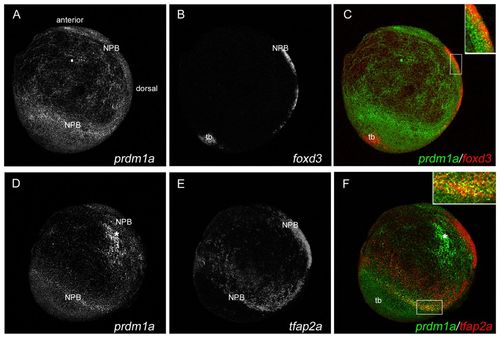
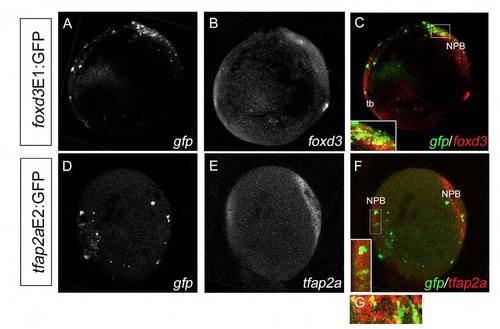
prdm1a is co-expressed with foxd3 and tfap2a at the NPB. Confocal micrograph projections of double fluorescent in situ hybridization (ISH) of 2-somite (11 hpf) wild-type (WT) zebrafish embryos for prdm1a with foxd3 (A-C) and for prdm1a with tfap2a (D-F). prdm1a (green) is co-expressed with both foxd3 (red, C) and tfap2a (red, F) at the NPB, as represented in yellow in the merged images (see insets in C and F). All images are lateral views with anterior to the top, dorsal to the right. Asterisk indicates non-specific staining. NPB, neural plate border; tb, tailbud. |
|
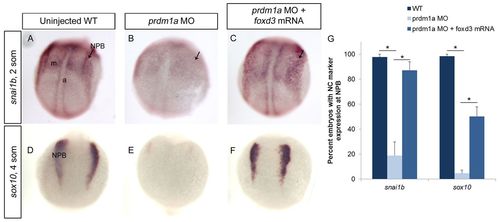
foxd3 mRNA rescues NCCs in prdm1a-deficient embryos. (A-F) ISH for neural crest markers snai1b (A-C) and sox10 (D-F) on 2- to 4-somite (11-12 hpf) uninjected zebrafish embryos (A,D), prdm1a morphants (B,E) and with prdm1a-MO co-injected with foxd3 mRNA (C,F). Dorsal view of WT embryos show neural crest expression at the NPB (arrows) for both snai1b and sox10, with snai1b also expressed in the adaxial cells and mesoderm. In prdm1a morphants, the expression is reduced at the NPB. However, after co-injection with foxd3 mRNA, the NPB expression is restored. All images are dorsal views, anterior to the top. a, adaxial cells; m, mesoderm. (G) Percentage of embryos expressing each marker. snai1b: WT, n (number of embryos exhibiting the phenotype in A out of the number of embryos examined)=81/84; prdm1a-MO, n=13/59; rescue, n=41/48. sox10: WT, n=156/160; prdm1a-MO, n=4/92; rescue, n=39/77. *P<0.05. Error bars indicate s.e.m. |
|
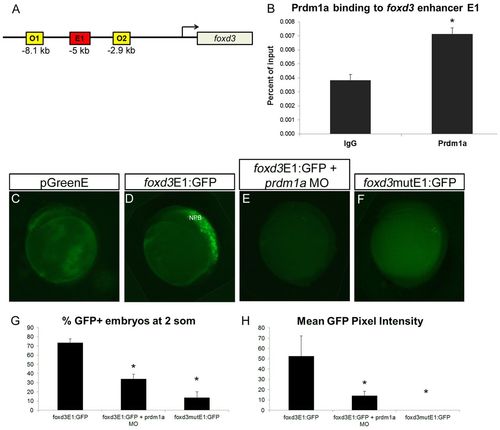
Prdm1a directly binds and activates a foxd3 enhancer at the NPB. (A) The zebrafish foxd3 locus showing one putative enhancer (E1) <5 kb upstream from the transcription start site that contains a binding sequence for Prdm1a, as well as the two off-target sites O1 and O2 used for ChIP. (B) Prdm1a ChIP pulls down foxd3 E1, which is enriched compared with the control IgG pulldown. (C-F) Lateral view of embryos injected with empty (no enhancer sequence) pGreenE GFP expression vector (C), foxd3 enhancer construct foxd3E1:GFP (D), foxd3E1:GFP with prdm1a-MO (E), and the foxd3 enhancer with a mutated Prdm1a binding site driving GFP as construct foxd3mutE1:GFP (F). Specific binding of Prdm1a to the foxd3 enhancer E1 is illustrated. Lateral views, anterior to the top. (G,H) The percentage of embryos expressing GFP (G) and the average pixel intensity of GFP (H). (G) foxd3E1:GFP, n=161/227; foxd3E1:GFP + prdm1a-MO, n=32/102; foxd3mutE1:GFP, n=29/166. (H) n=10 per condition. *P<0.05. Error bars indicate s.e.m. |
|
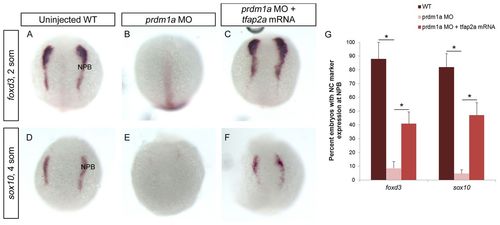
tfap2a mRNA rescues prdm1a-deficient neural crest. (A-F) Uninjected WT zebrafish embryos (A,D), prdm1a morphants (B,E) and prdm1a morphants co-injected with tfap2a mRNA (C,F) were subject to ISH for the neural crest markers foxd3 at 2-somites (A-C) and sox10 at 4-somites (D-F). WT embryos show defined expression of foxd3 and sox10 at the NPB, which is significantly decreased in prdm1a morphants. tfap2a mRNA injection rescues the neural crest in prdm1a-deficient embryos. Dorsal views, anterior to the top. (G) The percentage of embryos expressing each marker. foxd3: WT, n=49/58; prdm1a-MO, n=13/120; rescue, n=27/72. sox10: WT, n=45/56; prdm1a-MO, n=3/62; rescue, n=37/76. *P<0.05. Error bars indicate s.e.m. |
|
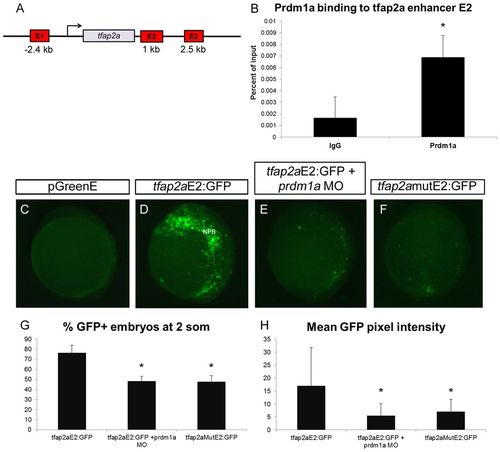
Prdm1a directly binds and activates a tfap2a enhancer at the NPB. (A) The zebrafish tfap2a locus showing the three putative enhancers E1, E2 and E3 (distance from the tfap2a transcription start site is indicated) that contain Prdm1a binding sequences and were analyzed by ChIP. (B) Prdm1a ChIP pulls down tfap2a E2, which is enriched compared with IgG. (C-F) Two-somite embryos injected with pGreenE GFP expression plasmid (C), tfap2aE2 driving GFP as construct tfap2aE2:GFP (D), tfap2aE2:GFP with prdm1a-MO (E), and tfap2aE2 with a mutated Prdm1a binding site driving GFP as construct tfap2amutE2:GFP (F). Specific binding of Prdm1a to an enhancer for tfap2a is illustrated. (G,H) The percentage of embryos expressing GFP (G) and the average pixel intensity of GFP (H). (G) tfap2aE2:GFP, n=55/68; tfap2aE2:GFP + prdm1a-MO, n=43/88; tfap2aE2mut:GFP, n=47/100. (H) tfap2aE2:GFP, n=23; tfap2aE2:GFP + prdm1a-MO, n=14; tfap2aE2mut:GFP, n=10. *P<0.05. Error bars indicate s.e.m. |
|
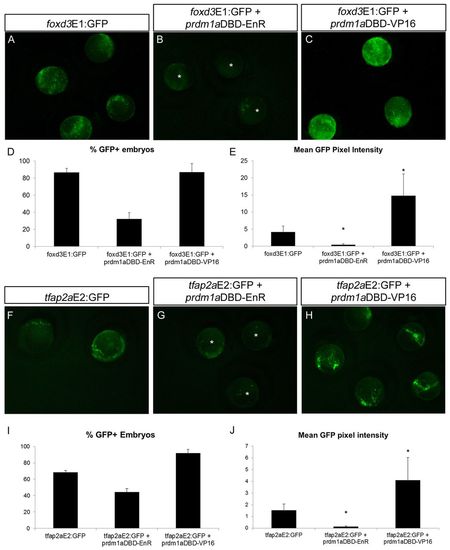
Prdm1a dominant activator and dominant repressor constructs directly regulate foxd3 and tfap2a enhancers. (A-C) Zebrafish embryos at the 2-somite stage expressing foxd3E1:GFP alone (A) or with prdm1aDBD-EnR dominant repressor (B) or prdm1aDBD-VP16 dominant activator (C). GFP expression is downregulated when foxd3E1:GFP is co-injected with prdm1aDBD-EnR (asterisks) and upregulated when the enhancer construct is co-expressed with prdm1aDBD-VP16. (D,E) The percentage of embryos expressing GFP (D) and GFP pixel intensity (E) in A-C. (D) foxd3E1:GFP, n=19/22; EnR, n=6/19; VP16, n=28/32. (E) foxd3E1:GFP, n=17; EnR, n=10; VP16, n=10. (F-H) Two-somite embryos expressing tfap2aE2:GFP with prdm1aDBD-EnR also exhibit downregulated GFP (G, asterisks), whereas when co-expressed with prdm1aDBD-VP16 they display increased GFP expression (H). (I,J) Percentage of GFP-positive embryos (I) and GFP pixel intensity (J) in F-H. (I) tfap2aE2:GFP, n=11/16; EnR, n=16/36; VP16, n=36/39. (J) tfap2aE2:GFP, n=11; EnR, n=14; VP16, n=12. *P<0.05. Error bars indicate s.e.m. |
|
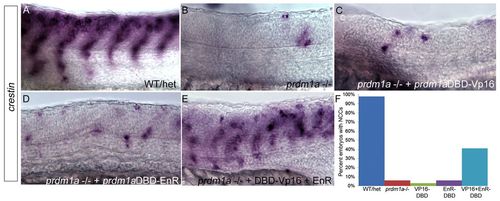
prdm1aDBD-VP16 and prdm1aDBD-EnR together rescue NCCs in prdm1a mutant embryos. (A-E) Lateral views of WT and prdm1a-/- zebrafish embryos at 24 hpf, dorsal to the top. ISH for crestin expression in WT and/or heterozygotes reveals migrating NCCs in the trunk (A). prdm1a mutant embryos have little NCC migration and most do not exhibit migration in more than seven somites (B). Injection of prdm1aDBD-VP16 (C) or prdm1aDBD-EnR (D) alone cannot rescue NCCs in prdm1a mutants. However, injection of prdm1aDBD-VP16 and prdm1aDBD-EnR together rescues crestin expression in prdm1a-/- embryos and NCCs of rescued animals are able to migrate similarly to WT (compare E with A), suggesting that Prdm1a must function as both a transcriptional activator and repressor for migratory NCCs to develop. prdm1a-/- embryos were identified by their curved tail, U-shaped somites and fin mesenchyme defects, and confirmed by genotyping. (F) The percentage of embryos with NCCs present in seven or more somites. Sample size: Prdm1a mutants alone (with NCCs in seven or more somites), n=7/106; Prdm1a mutants injected with Vp16, n=2/55; Prdm1a mutants injected with EnR, n=2/29; Prdm1a mutants injected with both prdm1a-DBD-Vp16 and EnR, n=52/125. |
|
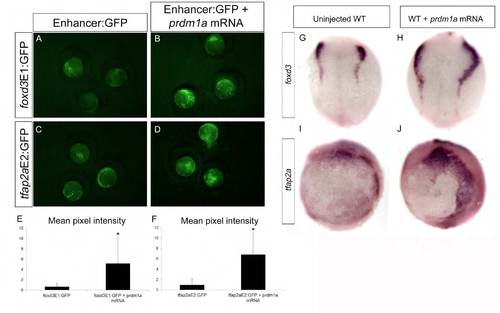
prdm1a overexpression leads to expansion of foxd3 and tfap2a enhancer reporters and endogenous expression. (A-F) prdm1a mRNA co-injected at the single-cell stage with foxd3E1:GFP (B,E; n=11) and tfap2aE2:GFP (D,F; n=25) and imaged at 2-somites produces increased GFP expression when compared with enhancer:GFP constructs alone (A,C). (G-J) prdm1a mRNA overexpression also increases the expression of endogenous foxd3 along the NPB (G,H; dorsal views; n=15) and expansion of the tfap2a expression domain at 2-somites (I,J; lateral views; n=10). |
|
Double fluorescent ISH shows mosaic colocalization of enhancer:GFP with endogenous foxd3 and tfap2a mRNA expression. (A-F) Double fluorescent ISH for gfp (A) and foxd3 (B) in foxd3E1:GFP-injected embryos (merge in C, lateral view) and for gfp (D) and tfap2a (E) in tfap2aE2:GFP-injected embryos (merge in F, dorsal view) at 2-somites demonstrate mosaic colocalization of enhancer:GFP with the endogenous gene along the NPB (insets in C,F). (G) tfap2a and gfp also colocalize in the most anterior NPB as shown in a lateral magnification from a separate WT embryo. NPB, neural plate border; tb, tailbud. |
|
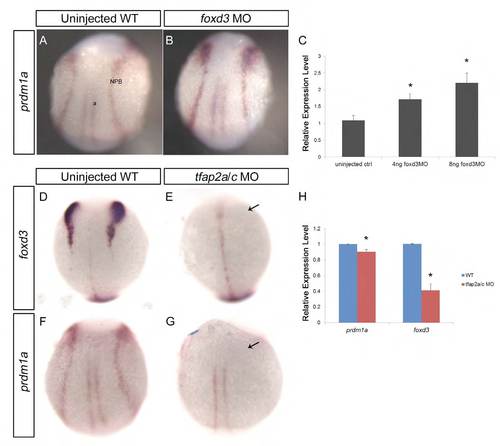
tfap2a expression is reduced by prdm1a-MO at the NPB. ISH was performed on uninjected WT embryos (A) and embryos injected with prdm1a-MO (B) for tfap2a at the 2-somite stage. Lateral view shows decreased tfap2a expression at the NPB in prdm1a morphants. |
|
foxd3 and tfap2a interact reciprocally with prdm1a at the NPB. (A,B) WT embryos (A) and embryos injected with 8 ng foxd3-MO (B) were fixed at 2-somites and ISH was performed for prdm1a. Dorsal view of embryos shows increased prdm1a expression in foxd3 morphants. (C) Embryos at 2-somites were also analyzed for prdm1a expression by qRT-PCR, showing a dose-dependent increase in prdm1a expression in response to foxd3-MO. (D-G) ISH for foxd3 (D,E) and prdm1a (F,G) was performed on uninjected WT (D,F) and tfap2a/tfap2c double-morphant embryos (E,G) at 2-somites. Dorsal views show the absence of foxd3 and prdm1a expression at the NPB in tfap2a/tfap2c morphants (arrows, E,G). (H) qRTPCR for prdm1a and foxd3 was also performed on WT and tfap2a/c morphants and showed decreased expression of both genes in the morphants. NPB, neural plate border; a, adaxial cells. *P<0.05. |
|
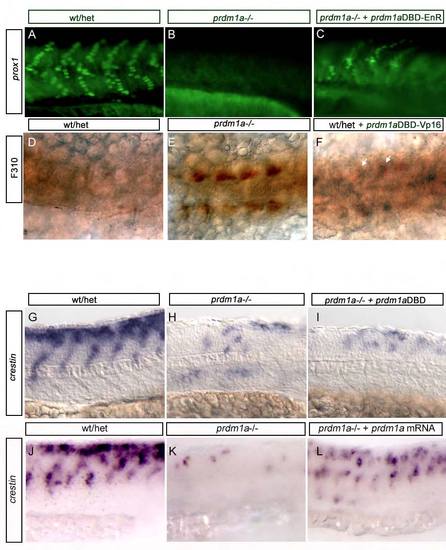
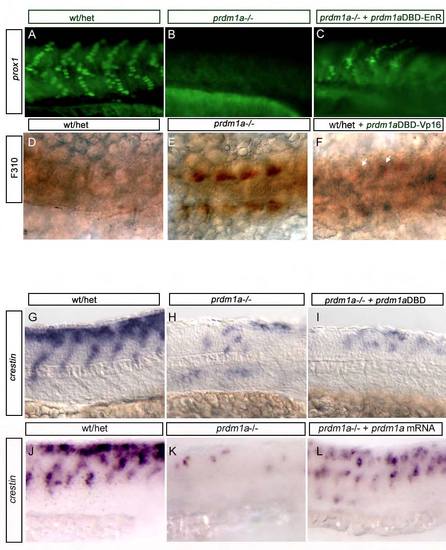
prdm1aDBD-VP16 activating and prdm1aDBD-EnR repressing constructs are functional. (A-C) Injection of prdm1aDBD-EnR construct into prdm1a–/– embryos rescues prox1 expression in slow-twitch muscle (10% rescued in C, n=4/20) as shown previously (von Hofsten et al., 2008). (D-F) Injection of prdm1aDBD-VP16 construct into WT embryos shows precocious F310 immunoreactivity (arrows in F), similar to what is observed in prdm1a–/– embryos (57% exhibit F310 immunoreactivity shown in F, n=47/82). (G-I) Injection of prdm1aDBD alone into prdm1a–/– embryos does not rescue crestin expression in prdm1a mutant embryos (100% of prdm1a mutants injected with prdm1aDBD alone exhibit reduced neural crest as in I, n=10/10). (J-L) crestin trunk expression in 24-hpf WT/het embryos (J), prdm1a–/– embryos (K) and prdm1a–/– embryos rescued with full- length prdm1a mRNA (L). |
|
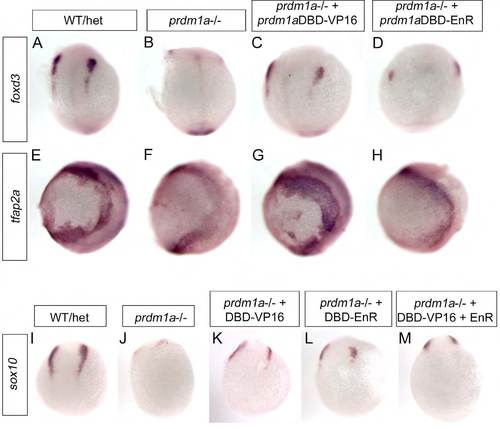
prdm1a dominant activator rescues foxd3 and tfap2a in prdm1a mutant embryos, and both activator and repressor forms rescue sox10. (A-H) Whole-mount ISH was performed on prdm1a–/– embryos injected with prdm1aDBD-VP16 activator or prdm1a DBD-EnR repressor at 2-somites for foxd3 (A-D, dorsal views) and tfap2a (E-H, lateral views). foxd3 and tfap2a are both decreased in prdm1a–/– embryos (B,F) compared with WT/het embryos (A,E). Injection of prdm1aDBD-VP16 partially rescues foxd3 (C) and expands tfap2a (G) at the NPB in prdm1a mutants, whereas prdm1aDBD-EnR does not rescue either foxd3 or tfap2a mRNA expression. (I-M) ISH for sox10 was performed on prdm1a–/– embryos injected with prdm1aDBD-VP16, prdm1aDBD-EnR or both combined at 4-somites. prdm1aDBD-VP16 (K), prdm1aDBD-EnR (L) and both combined (M) partially rescued sox10 expression in the NPB. Dorsal views. |