- Title
-
tgfbeta3 Regulation of Chondrogenesis and Osteogenesis in Zebrafish is Mediated Through Formation and Survival of a Subpopulation of the Cranial Neural Crest
- Authors
- Cheah, F.S., Winkler, C., Jabs, E.W., and Chong, S.S.
- Source
- Full text @ Mech. Dev.
|
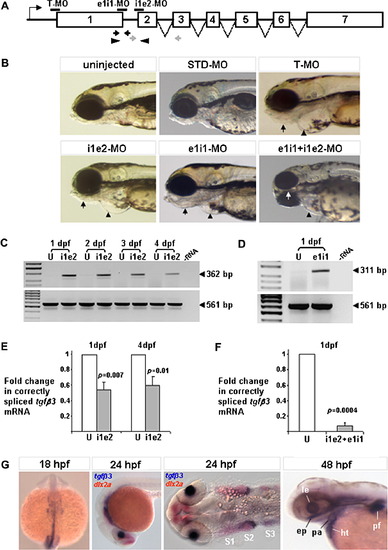
Suppression of tgfβ3 expression in the zebrafish embryo. (A) Gene knockdown via antisense Morpholino™ oligonucleotide mediated translation blocking (T-MO), and pre-mRNA splice inhibition (e1i1-MO and i1e2-MO). Morpholino™ annealing sites are indicated as horizontal black bars. Opposing gray and black arrows and arrowheads indicate positions of primer pairs used to detect presence of aberrantly-spliced and normally-spliced tgfβ3 mature transcripts, respectively. Introns are not drawn to scale. (B) Except for STD-MO injected embryos, all MO-injected larvae (96 hpf) have a smaller head, malformed lower jaw (arrows), and smaller heart with enlarged pericardial space (arrowheads). (C, D) RT-PCR of i1e2-MO and e1i1-MO injected embryos detects the presence of 362 bp and a 311 bp splice-modified tgfβ3 transcript fragments, respectively, which are not observed in uninjected controls (U). β-actin (561 bp) was used at a loading control. (E) Real-time relative quantitative PCR reveals a ∼50% reduction in normally-spliced tgfβ3 mRNA in i1e2-MO injected embryos compared to uninjected controls at 1 dpf and 4 dpf. (F) Co-injection of e1i1-MO with i1e2-MO suppresses correctly-spliced tgfβ3 mRNA by 93% compared to uninjected controls at 1 dpf. Standard deviations are based on three biological replicates. (G) Normal expression of tgfβ3 in a subpopulation of migrating neural crest cells at the 18-somite and 24 hpf stages, and in the ethmoid plate (ep), pharyngeal arch (pa), pectoral fin (pf), lens (le) and heart (ht) at 48 hpf. Red staining marks dlx2a-positive neural crest streams S1–S3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
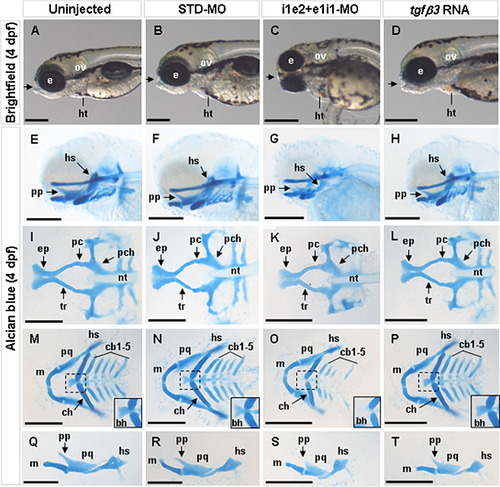
Effect of tgfβ3 knockdown and over-expression on craniofacial chondrogenesis in 4 dpf hatchlings (A–T). Whole-mount lateral (A–H, U–X), and flat-mount dorsal (I–L), ventral (M–P) and lateral (Q–T) views are shown. In the uninjected and STD-MO injected hatchlings, the mouth opening (arrow) is located anteriorly to both eyes (A, B), and the craniofacial cartilages (E, F) including the neurocranium (I, J) and pharyngeal arches (M, N) are distinct and normally developed. Lateral flat-mount of the first pharyngeal arch shows that the Meckel’s cartilage, palatoquadrate and hyosymplectic are normally formed (Q, R). In the i1e2 + e1i1 double morphant hatchling, the mouth opening is ventrally rotated (C), and the craniofacial cartilages appear under-developed (G), including a smaller neurocranium (K), hypoplastic posterior pharyngeal arches (O) and the absence of the basihyal (inset in O). Lateral flat-mount of the first pharyngeal arch shows that the Meckel’s cartilage, palatoquadrate and hyosymplectic are under-developed (S). In the tgfβ3 over-expressed hatchlings, no obvious abnormalities could be detected in the mouth opening (D) or the craniofacial cartilages (H, L, P, T). Insets in M-P show the zoom-in view of the dashed box area. bh, basihyal; cb1-5, ceratobranchial arches 1–5; ch, ceratohyal; e, eye; ep, ethmoid plate; hs, hyosymplectic; ht, heart; m, Meckel’s cartilage; nt, notochord; ov, otic vesicle; pa, pharyngeal arches; pc, polar cartilage; pch, parachordal; pf, pectoral fin; pp, pterygoid process of palatoquadrate; pq, palatoquadrate; tr, trabecula. Black horizontal scale bars, 200 μm. PHENOTYPE:
|
|
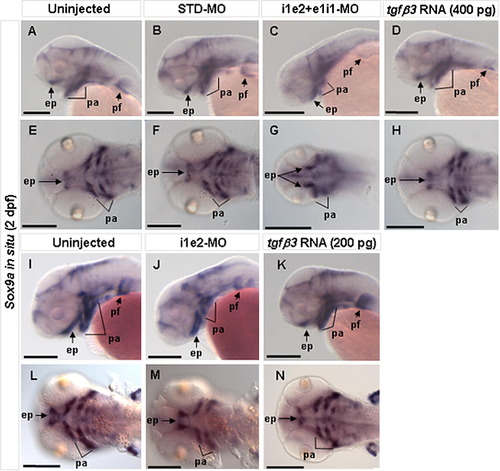
Effect of tgfβ3 knockdown and over-expression on craniofacial chondrogenesis in 2 dpf embryos (A–N). Whole-mount lateral (A–D, I–K), and flat-mount ventral (E–H, L–N) views are shown. Expression of the cartilage differentiation marker sox9a appeared similar in uninjected and STD-MO injected embryos (A, B, E, F, I, L). In the i1e2 + e1i1 double morphant, however, there is markedly reduced sox9a expression in the developing pharyngeal arches and absent expression in the medial ethmoid plate (C, G). Sox9a expression is also mildly reduced in tgfβ3 over-expressing embryos (400 pg mRNA), with absent expression in the medial ethmoid plate (D, H). In the i1e2 single morphant, there is still a reduced sox9a expression in the developing pharyngeal arches and absent expression in the medial ethmoid plate (J, M) compared to the uninjected control embryos (I, L). Similarly, sox9a expression is also weakly reduced in 200 pg tgfβ3 mRNA injected embryos (K, N). ep, ethmoid plate; pa, pharyngeal arches; pf, pectoral fin. Black scale bars, 200 μm. |
|
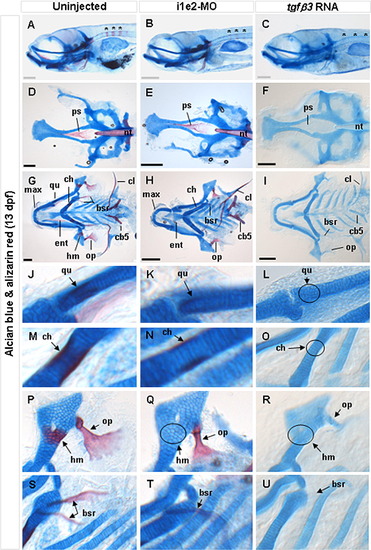
Effect of tgfβ3 knockdown and over-expression on craniofacial bone formation in 13 dpf hatchlings (A–U). Whole-mount lateral (A–C), flat-mount dorsal (D–F,) and flat-mount ventral (G–U) views are shown. In the uninjected 13 dpf hatchling (A), several ossified vertebrae (asterisks) are distinctly visible. These vertebrae are considerably less ossified in the knockdown morphant (B), and entirely undetectable in the tgfβ3 over-expressed hatchling (C). The parasphenoid of the neurocranium (ps) and rostral tip of the notochord (nt) are also normally ossified at 13 dpf (D). Alizarin red staining of these structures is marginally weaker in the knockdown morphant (E), and completely absent in the RNA injected hatchling (F). Pockets of ossification in the hyomandibula (hm) that are normally present at 13 dpf hatchling (G, P) are absent in both the knockdown morphant (H, Q) and over-expressed hatchling (I, R). In addition, the maxilla (max), opercle (op), entopterygoid (ent), ceratohyal (ch), branchiostegal rays (bsr), cleithrum (cl) and ceratobranchial arch 5 (cb5) are under-ossified in the knockdown morphant (H), and completely un-ossified in the over-expressed hatchlings (I). Compared with uninjected hatchling (J, M), the quadrate (qu) and ceratohyal (ch) which form the lateral palate and lower jaw, respectively, are under-ossified in the morphant (K, N), and completely un-ossified in the tgfβ3 over-expressing hatchling (circles in L, O). In addition, the hyomandibula (hm) is un-ossified (circle) and opercle (op) lacks the characteristic fan-shape structure in the morphant (Q) compared to uninjected hatchling (P). In the RNA injected hatchling (R), both the hyomandibula (circle) and opercle are completely un-ossified. The number of branchiostegal rays (bsr) is also reduced and under-ossified, and un-ossified in the knockdown morphant (T) and over-expressing hatchling (U), respectively. Grey and black horizontal scale bars represent 200 µm and 150 μm, respectively. PHENOTYPE:
|
|
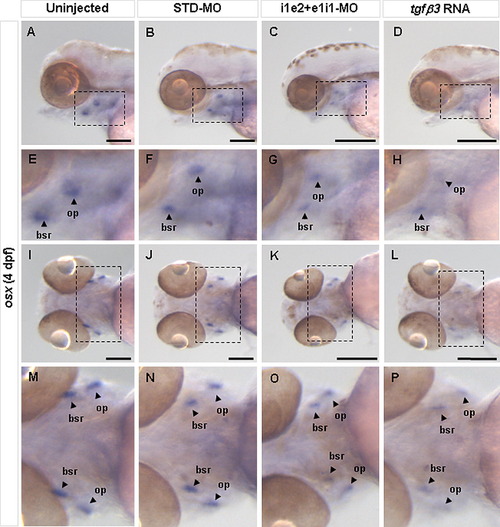
Effect of tgfβ3 knockdown and over-expression on osteoblast differentiation in 4 dpf larvae. Whole-mount lateral (A–D) and ventral (I–L) views are shown. E–H and M–P show the zoom-in view of the dashed box area in A–D and I–L, respectively. Analysis of the osteoblast differentiation marker osx in 4 dpf hatchlings reveals that, compared to uninjected (A, E, I, M) and STD-MO injected (B, F, J, N) hatchlings, both the double knockdown morphant (C, G, K, O) and tgfβ3 over-expressing hatchling (D, H, L, P) show markedly reduced expression in the pharyngeal arch region, most notably in the opercle (op) and branchiostegal ray (bsr). Black horizontal scale bars represent 200 μm. |
|
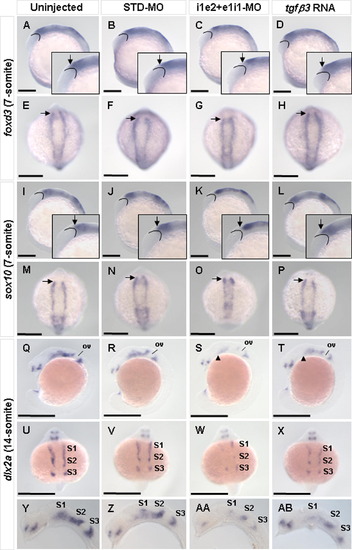
Effect of tgfβ3 knockdown and over-expression on induction and migration of neural crest cells. Whole-mount lateral (A–D, I–L, Q–T) and dorsal (E–H, M–P, U–X), and flat-mount lateral (Y–AB) views are shown. Expression of the pre-migratory neural crest markers foxd3 and sox10 was similar in the uninjected (inset in A, E, inset in I, M), STD-MO injected (inset in B, F, inset in J, N), and tgfβ3 over-expressing (inset in D, H, inset in L, P) 7-somite stage embryos. In the i1e2 + e1i1 double knockdown morphants, however, a reduction in expression domain at the anterior region of the bilateral rows of pre-migratory neural crest cells (arrow) was observed (inset in C, G, inset in K, O). Expression of the migratory neural crest marker dlx2a was similar in the uninjected (Q, U, Y) and STD-MO injected (R, V, Z) embryos at the 14-somite stage. In the double knockdown morphant (S, W, AA), dlx2 expression is visibly reduced in both intensity and domain, in all three neural crest streams especially around the anterior neural crest region of S1 (arrowhead) and S2. The tgfβ3 over-expressing embryo (T, X, AB) exhibits only a mild reduction in dlx2a expression. Arched lines demarcate posterior boundary of eye primordia. Arrows indicate anterior boundary of pre-migratory neural crest cells. Arrowheads indicate anterior boundary of neural crest stream 1. ov, otic vesicle; S1–S3, neural crest streams 1–3. Black horizontal scale bars, 200 μm. EXPRESSION / LABELING:
|
|
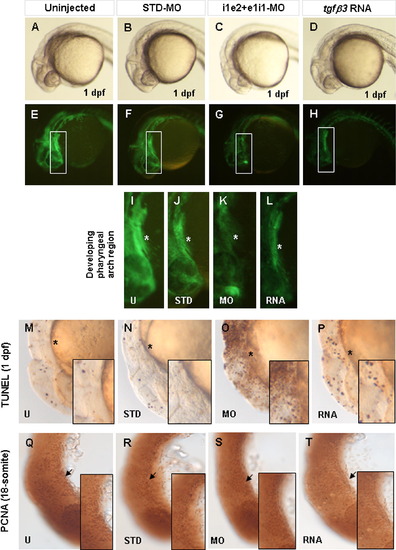
Effect of loss and over-expression of tgfβ3 on apoptosis and cellular proliferation. Whole-mount lateral (A–P) and flat-mount lateral (Q–T) views are shown in brightfield (A–D, M–T) and darkfield (E–L). The fli1a:gfp transgene is highly expressed in CNC-derived cells of the developing pharyngeal arch of uninjected (A, E, I) and STD-MO injected (B, F, J) transgenic 1 dpf embryos (asterisk). Injection of i1e2 and e1i1-MOs (C, G, K) or tgfβ3 RNA (D, H, L) leads to a visible reduction in transgene expressing cells. TUNEL analysis reveals a marked increase in apoptotic cells in double knockdown embryos (O) compared with uninjected (M) or STD-MO injected (N) embryos, while over-expressing embryos (P) showed a slight increase in apoptotic cells. PCNA immunohistochemistry of 18-somite stage embryos shows similar numbers of PCNA positive cells (arrow) in uninjected (Q), STD-MO injected (R), double knockdown morphant (S), and over-expressing (T) embryos. Insets in M–T show the zoom-in view of the developing pharyngeal arch region. EXPRESSION / LABELING:
PHENOTYPE:
|
|
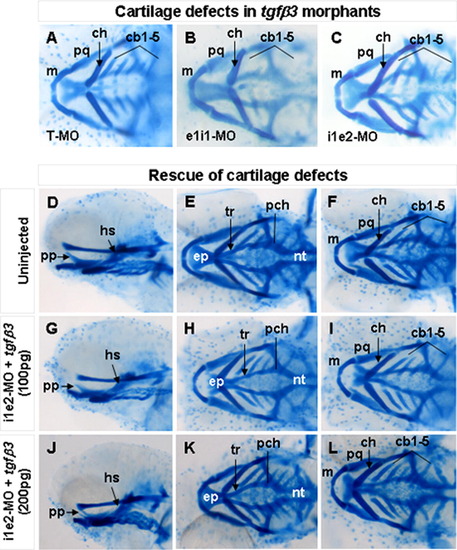
Craniofacial cartilage phenotypes in 4 dpf morphents injected with different tgfβ3-targeting antisense MOs and their rescue by co-injection of tgfβ3 mRNA. Whole-mount ventral (A–C, F, I, L), lateral (D, G, J), and dorsal (E, H, K) views are shown. Similar cartilage defects are detected in hatchlings injected with translation blocking T-MO (A), splice inhibition elit-MO (B) and i1e2-MO (C). Compared with uninjected wild-type hatchlings (D–F), co-injection of 100 pg of tgfβ3 mRNA rescued the cartilage defects in 27% of the i1e2 MO-injected hatchling (G–I), whereas 33% of the i1e2 MO-injected hatchlings were rescued by co-injection of 200 pg of tgfβ3 mRNA (J–L), cbl-5. ceratobranchial arches 1–5; ch, ceratohyal; ep, ethmoid plate; hs, hyosymplectic; m, Meckel’s cartilage; nt, notochord; pch, parachordal; pp, pterygoid process of palatoquadrate; pq, palatoquadrate; and tr, trabecuta. PHENOTYPE:
|
|
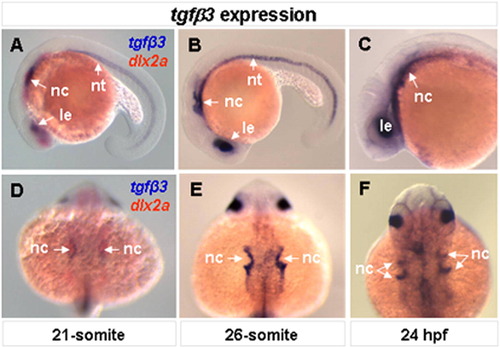
Embryonic expression of tgfβ3. Whole-mount lateral (A–C) and dorsal (D–F) views are shown. At the 21-somite stage, tgfβ3 is expressed in the lens, migrating neural crest cells and notochord (A, D). Double-color in situ hybridization with known migrating neural crest marker, dlx2a, shows that tgfβ3 is expressed in a subpopulation of the migrating neural crest cells (A, D). The expression of tgfβ3 in the migrating neural crest cells intensifies in streams 2 and 3 at the 26-somrte stage (B, E) and in 24 hpf (C, F) embryos. EXPRESSION / LABELING:
|
Reprinted from Mechanisms of Development, 128(7-8), Cheah, F.S., Winkler, C., Jabs, E.W., and Chong, S.S., tgfbeta3 Regulation of Chondrogenesis and Osteogenesis in Zebrafish is Mediated Through Formation and Survival of a Subpopulation of the Cranial Neural Crest, 329-344, Copyright (2010) with permission from Elsevier. Full text @ Mech. Dev.