- Title
-
Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants
- Authors
- Sullivan-Brown, J., Schottenfeld, J., Okabe, N., Hostetter, C.L., Serluca, F.C., Thiberge, S.Y., and Burdine, R.D.
- Source
- Full text @ Dev. Biol.
|
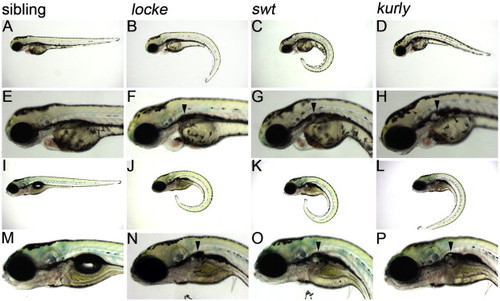
locke, swt and kurly mutants develop pronephric cysts. (A–H) 3 dpf, (I–P) 5 dpf. At 3 dpf, locke, swt and kurly mutants are easily identified by their “curly-tail down” phenotype. Panels E–H are higher magnification images of panels A–D, showing the cystic dilations visible under light microscopy. At 5 dpf, the cystic dilations have increased in size shown in panels I–L and magnified in panels M–P. The black arrowheads mark the location of the cysts, posterior to the eye and ear. In general, locke mutants have smaller cysts than swt or kurly. PHENOTYPE:
|
|
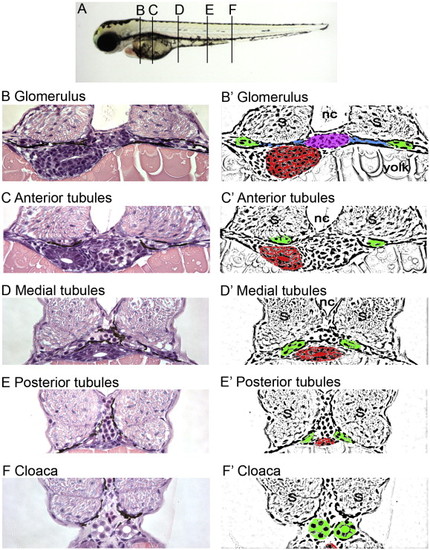
Histological and schematic representations of the pronephros along the anterior to posterior axis. (A) Depiction of the corresponding regions referred to in the text. (B, C, D, E, F) Histological sections stained with hematoxylin and eosin. Pictures taken with a 40x objective lens. (B′, C′, D′, E′, F′) Schematic diagrams highlighting regions of interest. (B, B′) Glomerulus: The glomerulus (pink) is found ventral to the notochord (nc) and medial to either somite (s). Connecting to the glomerulus are tubules that extend laterally (blue). The tubules then turn at the edge of the somites and extend toward the posterior (green). Also shown is the gut (red). (C, C′) Anterior tubules: The region designated as the anterior tubules is slightly posterior to the glomerulus region in which the viscera can be observed. (D, D′) Medial tubules: In this region, the gut (red) and tubules (green) are positioned toward the midline and ventral to the notochord (nc). (E, E′) Posterior tubules: The gut has become smaller and the tubules (green) are positioned more medially. (F, F′) Cloaca: This is the most posterior section before the tubules fuse into a single opening outside the body. |
|
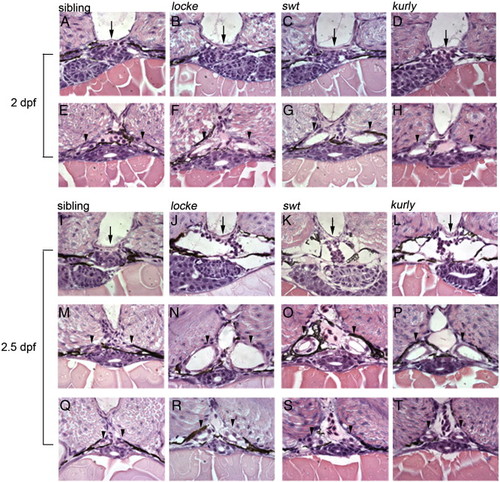
Temporal and spatial analysis of cystic kidneys in zebrafish mutants. (A–H) 2 dpf; (I–T) 2.5 dpf. At 2 dpf, the glomerulus (arrows, A–D) appears intact in the mutant embryos; however, the medial tubules have become dilated (arrowheads, E–H). At 2.5 dpf, the glomerulus (arrows, I–L) and surrounding Bowman's space have become enlarged, compressing the podocytes in the mutants. The medial tubules are grossly dilated (arrowheads, M–P). Interestingly, the posterior tubules are less dilated, approaching wild-type size (arrowheads, Q–T). All pictures are 4 μm JB-4 plastic sections, stained with hematoxylin and eosin and taken with a 100x oil objective lens. |
|
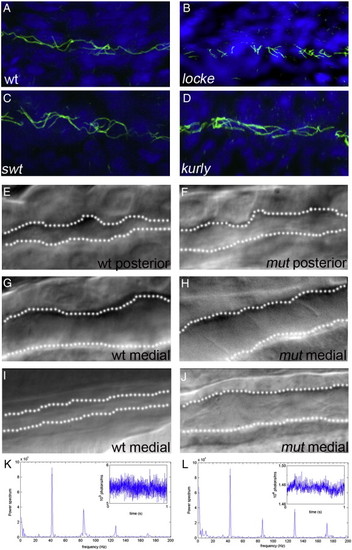
Early pronephric cyst phenotypes. (A–D) Acetylated tubulin staining in the pronephric tubules at 27 hpf. Cilia were detected using an anti-acetylated tubulin antibody (green) and counterstained with the nuclear marker Hoechst (blue). Cilia appeared grossly normal in swt and kurly, but are shorter in locke when compared to wild-type siblings. (E–J) DIC microscopy images of the pronephric tubules in wild-type siblings and kurly mutants. Lumen sizes in posterior tubules from 26–30 hpf are similar in both wild-type siblings (E) and mutant embryos (F). Lumens in the medial tubules are larger in both wild-type siblings (G) and mutant embryos (H) when compared in the posterior regions (E, F). By 2 dpf, there is a clear dilation in the medial tubules of mutant embryos (J) as compared to wild-type siblings (I). (K, L) Infra-red scattering measurements demonstrate that the frequency of cilia movement in kurly mutants (L) is similar to the frequency observed in wild-type sibling embryos (K) (∼ 42 Hz). Both spectra show several peaks. The measurements demonstrate that the frequency of cilia movement in kurly mutants (L) is similar to the frequency observed in wild-first peak in each spectrum corresponds to the fundamental frequency of cilia movement. The subsequent peaks corresponds to the harmonics of the fundamental frequency (∼ n * 42 Hz with n = 1, 2 and 3). Panels E–J were taken with a 60x objective water immersion lens using two different cameras. Panels E–H were taken with an iXon camera (Andor); pixel size 16 * 16 μm. Panels I and J were taken with a Luca camera (Andor); pixel size of 10 * 10 μm. Because of differences in pixel size, luminal size in panels E–H cannot be directly compared to panels I and J. |
|
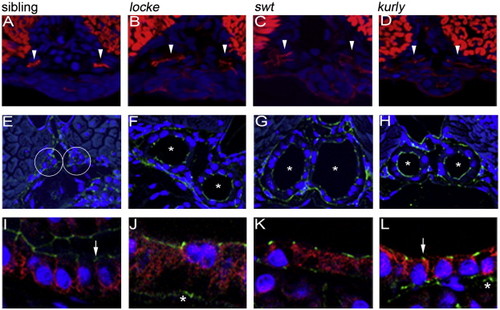
Na+/K+ATPase localization is disrupted in cystic tissues, while apical polarity is maintained. Apical localization of F-actin (red) observed in 2 dpf wild-type siblings (A) is maintained in the medial tubules (white arrowheads) of mutants (B–D). The somites and gut are also stained by phalloidin (red). Correct apical localization of ZO-1 (green) is observed in 4 dpf wild-type sibling (E) and mutant (F–H) embryos. Sibling tubules are outlined by a white circle, and mutant tubules are distinguished by asterisks. Basolateral localization of the Na+/K+ATPase (red) observed in wild-type siblings (I) is altered in cystic mutants at 4 dpf (J–L). Note in panels J–L that Na+/K+ATPase is adjacent to apical ZO-1 expression (green; indicated by white arrows) which is not observed in wild-type (I). All confocal images were taken with a 40× water objective lens. Panels A–D are cryosections; panels E–L are plastic sections. Nuclei (blue) are stained with either DRAQ5 (A–D) or Hoechst (E–L). ZO-1 staining basal to the pronephros comes from a different tissue (asterisks in panels K and L) and does not reflect an alteration in ZO-1 within the pronephros. |
|
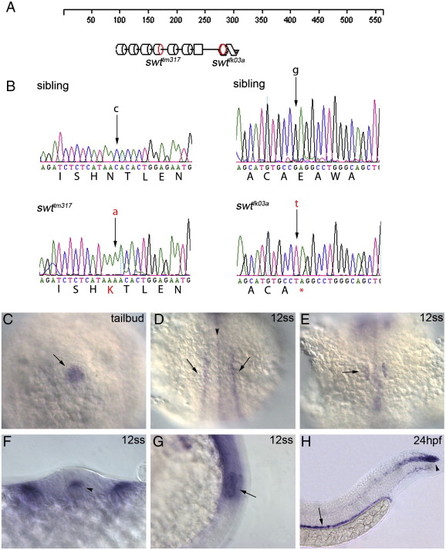
swt encodes lrrc50, a novel gene required for cilia motility. (A) Schematic diagram of the LRRC50 protein highlighting the location of tm317 and fk03a mutations. LRRC50 contains 6 predicted leucine rich repeats (cylinders), followed by a coiled-coil domain (spiral); however, the functions of these motifs in Swt are unknown. (B) Sequence traces of wild-type sibling and mutant DNA, showing the mutations in each allele. The swttm317 allele creates a missense mutation changing AAC (ASN) to AAA (LYS) at amino acid position 172 in the leucine rich repeat region. The swtfk03a allele creates a nonsense mutation changing GAG (GLU) to TAG (stop codon) at amino acid position 259 after the leucine rich repeats before the coiled-coil domain. swt RNA is expressed in cells that contain cilia: (C) Kupffer′s vesicle (arrow); (D) neural tube staining (arrowhead) and bilateral intermediate mesoderm staining (arrows); (E) otic vesicle (arrow); (F) cross-section showing expression i cells that contain cilia: (C) Kupffer′s vesicle (arrow); (D) neural tube staining (arrowhead) and bilateral intermediate mesodn the floorplate of the neural tube (arrowhead); (G) lateral view of otic vesicle expression (arrow); (H) pronephric duct (arrow, dorsal to the yolk extension) and chordo-neural hinge expression (arrowhead, at the tip of the tail). Embryo in panel C is at tailbud stage (dorsal view of posterior), panels D–G are at the 12 somite stage, and panel H is at 24 hpf. In panels D and E, anterior is up. |
|
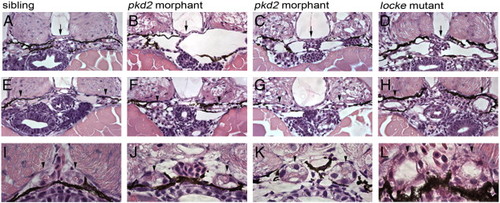
pkd2 morphants develop glomerular dilations, but do not become dilated in the tubular region of the nephron. (A–D) Glomerular region comparison at 3 dpf of a wild-type sibling embryo (A), pkd2 morphants (B, C) and a locke mutant embryo (D). Black arrows mark the glomerulus, which is clearly dilated in both pkd2 morphants and locke mutant embryos. (E–H) Anterior tubule region, immediately posterior to the glomerulus; although the tubules exhibit dilation in locke mutant embryos (H), the tubules in pkd2 morphants (F, G) are not enlarged and resemble wild-type sibling tubules (E). (I–L) Medial tubule region; unlike locke mutant embryos (L), the lumens of the medial tubules are not dilated in pkd2 morphants (J, K), similar to wild-type siblings (I). Black arrowheads indicate tubules. All pictures are from JB-4 plastic sections, stained with an H&E dye, and taken with 40x lens (A–H) and 100x oil lens (I–L). PHENOTYPE:
|
Reprinted from Developmental Biology, 314(2), Sullivan-Brown, J., Schottenfeld, J., Okabe, N., Hostetter, C.L., Serluca, F.C., Thiberge, S.Y., and Burdine, R.D., Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants, 261-275, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.