Fig. S3
|
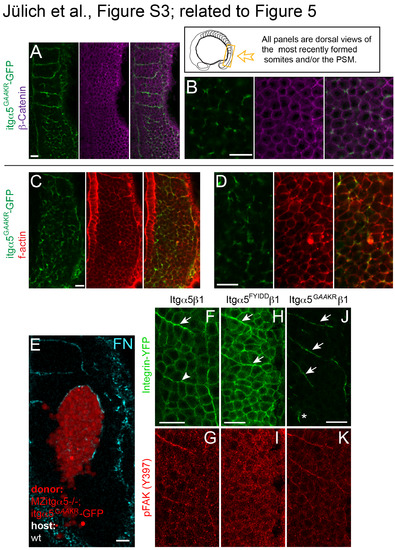
Activated Itgα5 induces FN matrix production and actin colocalization but does not affect β-Catenin localization. Localization of Itgα5GAAKR-GFP (green) and β-Catenin (magenta) at low (A) and high (B) resolution. Expression of Itgα5GAAKRGFP in MZ itgα5-/- embryos does not cause loss of adhesions associated with Integrin clustering (n=19 embryos). Localization of Itgα5GAAKR-GFP (green) and f-actin via phalloidin staining (red) at low (C) and high (D) resolution. As Integrin clustering normally colocalizes with thick cortical f-actin along somite borders (Jülich et al., 2005; Jülich et al., 2009), activated Itgα5GAAKR-GFP clustering colocalizes with higher levels of f-actin (n=16 embryos).(E) Itgα5GAAKR-GFP mRNA was injected into MZ itgα5-/- embryos and late blastula cells were transplanted into late blastula wild-type embryos. Clones were analyzed in the paraxial mesoderm of 10-13 somite stage embryos (n=85 embryos). Of 51 clones in the PSM, 37% showed FN along the surface of the clone, while 53% of clones in the somitic mesoderm exhibited FN on the surface of the clone. Scale bars are 20 µm. FAK phosphorylation follows Itgα5 clustering and ligand binding. Focal Adhesion Kinase (FAK) mediates Integrin outside-in signaling in response to FN binding and is necessary for normal FN fibrillogenesis (Ilic et al., 2004). In addition, expression of a dominant negative FAK perturbs Xenopus somite morphogenesis (Kragtorp and Miller, 2006). in vitro, phosphorylation of FAK on Y397 [pFAK(Y397)] is indicative of full FAK activation via cell anchoring and application of mechanical tension (Friedland et al., 2009). pFAK(Y397) is observed along zebrafish somite boundaries and is significantly reduced in itgα5-/- embryos (Crawford et al., 2003; Henry et al., 2001; Koshida et al., 2005). Using immunolocalization for pFAK(Y397), we sought to determine the timing of FAK activation relative to Itgα5 clustering and ligand binding during border morphogenesis. (F and G) We performed immunohistochemistry for pFAK(Y397) on fixed MZ itgα5-/- embryos rescued with Itgα5-GFP. Higher pFAK(Y397) phosphorylation can be observed along the somite boundaries relative to the basal level of pFAK(Y397) present with the presomitic mesoderm. We compared the pFAK(Y397) pattern with that of Itgα5-GFP on nascent somite borders and observed some Itgα5-GFP clustering that did not colocalize with pFAK(Y397). The arrowhead indicates a region of Itgα5 clustering that precedes the increase in pFAK(Y397) while the arrow designates a more mature somite border. These data suggest both that clustering precedes the onset of FAK phosphorylation and that FAK is phosphorylated only after Itgα5 adopts the extended conformation and binds FN. To examine this hypothesis, we performed immunohistochemistry for pFAK(Y397) on MZ itgα5-/- embryos expressing the ligand binding deficient Itgα5FYLDD-GFP. (H and I) confocal images of MZ itgα5-/- embryos expressing Hs Itgα5FYIDDβ1-YFP. Arrows indicate transient segmental Integrin clustering. No elevated levels of pFAK(397) are associated with Hs Itgα5FYIDDβ1 clustering. While Itgα5FYLDD-GFP transiently clusters in a segmental pattern, we observed no colocalization of elevated pFAK(Y397) above background. These data suggest that Itgα5 first clusters and adopts the extended “active” conformation, then binds FN, and then initiates outside-in signaling via FAK phosphorylation. The in vitro requirement for FAK in FN fibrillogenesis (Ilic et al., 2004) suggests that FAK phosphorylation induces a positive feedback that is necessary for FN matrix remodeling. (J and K) confocal images of MZ itgα5-/- embryos expressing activated Hs Itgα5GAAKRβ1-YFP. Arrows indicate Integrin clustering at somite borders. No elevated levels of pFAK(397) are associated with inappropriate Itgα5GAAKRβ1 clustering in the anterior presomitic mesoderm (asterisk). This lack of FAK activation, and hence failure to establish the positive feedback, correlates with the ephemeral dynamics of the associated FN matrix (Figure 5). In all panels, anterior is up and lateral is right. We examined pFAK in 104 embryos expressing wild-type itgα5 (7 experiments), 64 embryos expressing ligand binding-deficient itgα5 (6 experiments) and 57 embryos expressing activated itgα5 (5 experiments). Scale bars are 20 µm. |
Reprinted from Developmental Cell, 34(1), Jülich, D., Cobb, G., Melo, A.M., McMillen, P., Lawton, A.K., Mochrie, S.G., Rhoades, E., Holley, S.A., Cross-Scale Integrin Regulation Organizes ECM and Tissue Topology, 33-44, Copyright (2015) with permission from Elsevier. Full text @ Dev. Cell