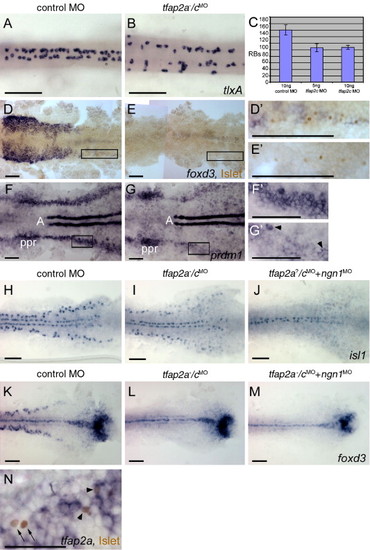
Rohon–Beard sensory neurons (RBs) are reduced but not absent in tfap2a/c deficient embryos. (A, B) Dorsal trunk views of 20 hpf embryos processed to reveal tlxA expression, anterior to the left. RBs are abundant in panel A, a wild-type or tfap2a mutant embryo, but moderately reduced in panel B, a tfap2a-/cMO embryo. Note that RBs are abnormally distributed in the latter, suggestive of abnormal patterning in the dorsal neural tube. (C) The average number of tlxA-positive cells in the dorsal spinal cord at 20 hpf. Error bars indicate standard deviation. Left column, embryos derived from tfap2a heterozygous mutants and injected with control MO, n = 23 embryos. Middle column, tfap2a mutants injected with 5 ng tfap2c e3i3 MO, n = 5 embryos. Right column, tfap2a mutants injected with 10 ng tfap2c e3i3 MO, n = 8 embryos. (D, E) Dorsal views of flat mounted 12 hpf embryos stained for anti-Islet immunoreactivity (brown, nuclear) and foxd3 mRNA (purple, cytoplasmic), anterior to the left. Premigratory neural crest (pnc) cells expressing foxd3 and RBs expressing anti-Islet IR are present in panel D, a control embryo. RBs and pnc cells are intermingled, as seen at higher magnification in panel D′ (corresponds to box in panel D). In panel E, a presumed tfap2a-/cMO embryo, foxd3 expression is absent while RBs are still present, as shown at higher magnification in panel E′ (11 of 40 injected embryos derived from tfap2a heterozygous mutants). (F, G) Dorsal views of flat mounted 11 hpf embryos processed to reveal prdm1 expression, anterior to the left. In panel F, a control embryo, prdm1 expression is detected in the pre-placodal region (ppr), in the neural plate boundary in the trunk (panel F′ corresponds to box in panel F), and in adaxial cells (A). (G) In a presumed tfap2a-/cMO embryo, prdm1 is expressed in scattered cells at the neural plate border (arrowheads) (panel G′ corresponds to box in panel G) (7 of 29 injected embryos derived from tfap2alow heterozygotes). (H–M) Dorsal trunk views of flat mounted 12.5 hpf embryos. isl1 (H) and foxd3 (K) expression shown in control embryos. (I) In the presumed tfap2a-/cMO embryos, isl1 expression is moderately reduced (16 of 56 injected embryos derived from tfap2alow heterozygotes), while foxd3 expression is absent (panel L, 12 of 58 injected embryos derived from tfap2alow heterozygotes). (J) In an embryo derived from tfap2a heterozygous mutant parents and co-injected with tfap2c e3i3 MO and ngn1 MO, isl1 expression in lateral neural plate, indicating RBs, is absent (48 of 51 tfap2c MO and ngn1 MO injected embryos). (M) In a presumed tfap2a mutant injected with tfap2c MO and ngn1 MO, foxd3 expression is absent (M, 16 of 53 injected embryos derived from tfap2alow heterozygotes). (N) Dorsal view of a 12.5 hpf embryo processed to reveal anti-Islet IR (brown, nuclear) and tfap2a mRNA (purple, cytoplasmic), anterior to the left. Some RBs that are positive for anti-Islet IR also express tfap2a (arrowheads). There are also RBs that are devoid of tfap2a expression in the cytoplasm (arrows). Scale bars: (A–M), 100 μm; (N), 50 μm.
|

