- Title
-
The Nature and Origin of Synaptic Inputs to Vestibulospinal Neurons in the Larval Zebrafish
- Authors
- Hamling, K.R., Harmon, K., Schoppik, D.
- Source
- Full text @ eNeuro
|
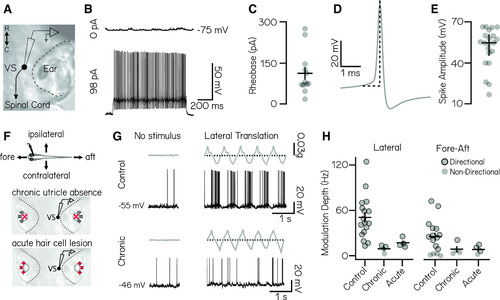
Vestibulospinal neurons encode utricle-derived body translation. |
|
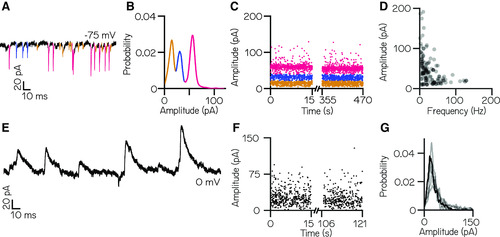
Larval zebrafish vestibulospinal neurons receive dense spontaneous synaptic input. |
|
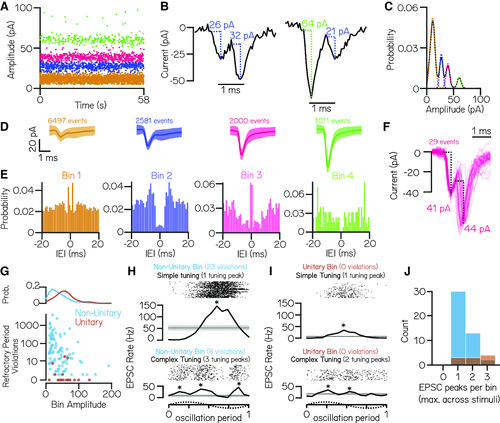
EPSC events within the same amplitude bin predominantly reflect multiple neuronal inputs. |
|
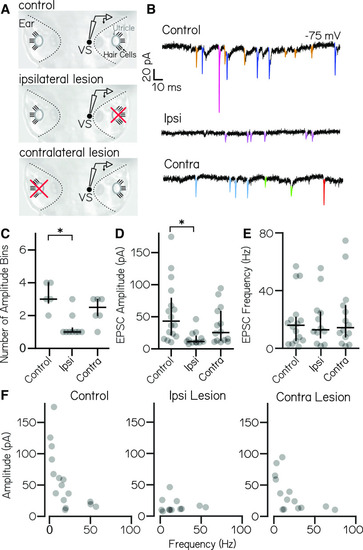
High-amplitude spontaneous excitatory inputs originate in the ipsilateral ear. |
|
Inhibitory current inputs have ipsilateral and contralateral vestibular sensory origins. |
|
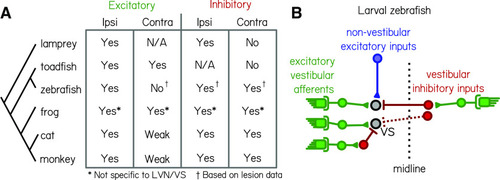
Comparative synaptic architecture of zebrafish vestibulospinal neurons. |