- Title
-
Teneurin trans-axonal signaling prunes topographically missorted axons
- Authors
- Spead, O., Moreland, T., Weaver, C.J., Costa, I.D., Hegarty, B., Kramer, K.L., Poulain, F.E.
- Source
- Full text @ Cell Rep.
|
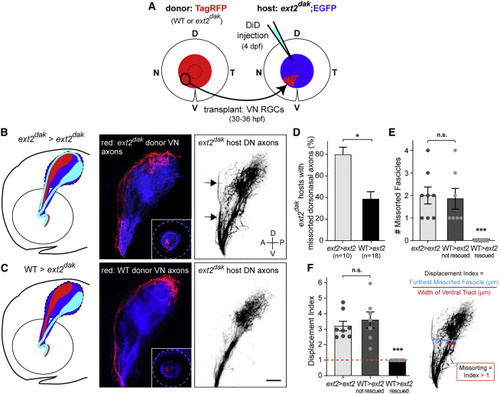
HS-mediated trans-axonal signaling corrects pre-target topographic sorting errors (A) Ventronasal (VN) RGCs of an isl2b:TagRFP donor embryo (WT or ext2dak mutant lacking HS) were topographically transplanted between 30 and 36 hpf into the VN retina of an isl2b:EGFP ext2dak mutant host. Pre-target sorting of host dorsonasal (DN) axons along the optic tract was assessed by injecting DiD into the host DN retina at 4 dpf. (B and C) Diagrams and lateral views of donor VN axons (red) and host DN axons (inverted images) along the optic tract of ext2dak hosts at 4 dpf. (B) Ext2dak DN axons missort along the dorsal branch of the optic tract in ext2dak hosts transplanted with ext2dak VN RGCs (arrows). (C) Missorting of ext2dak DN axons is corrected after transplanting WT VN RGCs. Scale bar: 50 μm. (D) Quantifications of the percentage of transplanted ext2dak hosts with missorted DN axons at 4 dpf. One-sided chi-squared test with Yates’s correction, X2 (1, N = 28) = 2.872, p = 0.0451. (E) Number of missorted DN axon fascicles in ext2dak hosts with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0005. (F) Quantification of the displacement index in ext2dak hosts with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. Error bars: standard errors. See also Figure S1. |
|
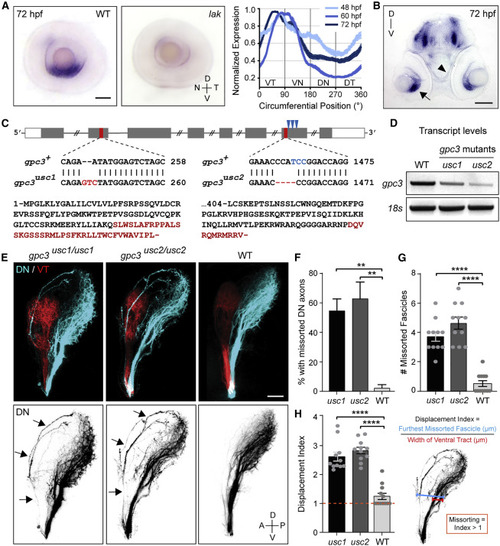
gpc3 is selectively expressed by ventral RGCs and is required for pruning missorted dorsal axons (A) Lateral views of eyes dissected from WT and lak mutant embryos lacking RGCs and stained for gpc3 by ISH at 72 hpf. Gpc3 is selectively detected in ventral RGCs. Scale bar: 50 μm. N, nasal; T, temporal; D, dorsal; V, ventral. Quantification of gpc3 expression in the RGC layer at 48, 60, and 72 hpf reveals that gpc3 expression is restricted to ventral RGCs at the time missorted dorsal axons get pruned. (B) Coronal section of an embryo stained for gpc3 by ISH at 72 hpf. Gpc3 is strongly detected in ventral RGCs (arrow) but not along the optic tract (arrowhead). Scale bar: 100 μm. (C) Schematic of gpc3 gene and corresponding mutations in gpc3usc1 and gpc3usc2 mutants. Red bars indicate the position of mutations in exons 2 and 7. Blue arrows indicate the three codons encoding HS attachment sites in exon 7. Protein sequences show the missense amino acids and premature stop generated in each allele. (D) Analysis of gpc3 transcript levels in WT and gpc3 mutants by RT-PCR at 4 dpf. Gpc3 transcripts are reduced by 48.4% and 68.4% in gpc3usc1 and gpc3usc2 mutants, respectively, indicating a non-sense-mediated decay of gpc3 mRNA in gpc3 mutants. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.005. (E) Pre-target sorting of DN and ventrotemporal (VT) retinal axons along the optic tract at 4 dpf. DN axons missort along the dorsal branch in gpc3 mutants (arrows). Scale bar: 50 μm. (F) Percentage of larvae with missorted DN axons at 4 dpf. Chi-squared test, X2 (2, N = 51) = 11.58, p = 0.0031. (G) Number of missorted DN axon fascicles in mutant larvae with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. (H) Quantification of the displacement index in mutant larvae with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. n = 11 gpc3usc1 and 11 gpc3usc2. Error bars: standard errors. See also Figures S2–S4. |
|
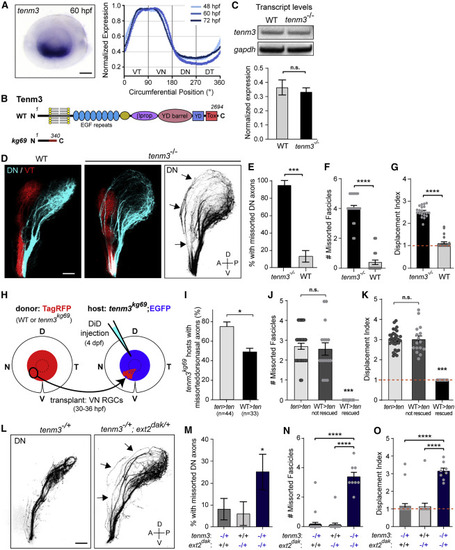
tenm3 is required for pruning topographically missorted dorsal axons (A) tenm3 ISH staining is selectively detected in the ventral RGC layer in the retina throughout development. Scale bar: 50 μm. (B) Schematic of Tenm3 protein structure in WT and tenm3kg69 mutants. Tox, toxin-like domain; YD, tyrosine-aspartate repeat; βprop, β-propeller. (C) Analysis of tenm3 transcript levels by RT-PCR in WT and tenm3 mutants at 4 dpf. Unpaired two-tailed t test p = 0.75. Error bars: standard errors. (D) Pre-target sorting of DN and VT retinal axons along the optic tract at 4 dpf. Missorted DN axons are maintained along the dorsal branch of the tract in tenm3 mutants (arrows). Scale bar: 50 μm. (E) Percentage of larvae with missorted DN axons at 4 dpf. One-sided chi-squared test with Yates’s correction, X2 (1, N = 47) = 23.30, p < 0.0001. (F) Number of missorted DN axon fascicles in phenotypic larvae. Unpaired one-tailed t test, p ˂ 0.0001. (G) Quantification of the displacement index in larvae with sorting defects. Unpaired one-tailed t test, p ˂ 0.0001, n = 20 mutants. (H) VN RGCs of an isl2b:TagRFP donor embryo (WT or tenm3 mutant) were topographically transplanted between 30 and 36 hpf into the VN retina of an isl2b:EGFP tenm3 mutant host. Pre-target sorting of host DN axons was assessed by injecting DiD into the host DN retina at 4 dpf. (I) Quantifications of the percentage of transplanted tenm3 mutant hosts with missorted DN axons. One-sided chi-squared test with Yates’s correction, X2 (1, N = 77) = 4.641, p = 0.0156. (J) Number of missorted DN axon fascicles in tenm3 mutant hosts with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. (K) Quantification of the displacement index in tenm3 mutant hosts with sorting defects. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. Error bars: standard errors. (L) Pre-target sorting of DN retinal axons along the optic tract at 4 dpf. DN axons missort along the dorsal branch of the tract in tenm3−/+; ext2dak/+ larvae (arrows) but not in tenm3−/+ heterozygotes. Scale bar: 50 μm. (M) Percentage of tenm3−/+, ext2dak/+, and tenm3−/+; ext2dak/+ larvae with missorted DN axons at 4 dpf. One-sided chi-squared test, X2 (1, N = 74) = 3.274, p = 0.035. (N) Number of missorted DN axon fascicles in phenotypic larvae. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. (O) Quantification of the displacement index. One-way ANOVA with Tukey’s post-hoc test, p ˂ 0.0001. n = 25 tenm3−/+, 17 ext2dak/+, and 32 tenm3−/+; ext2dak/+. Error bars: standard errors. See also Figure S6. |
|
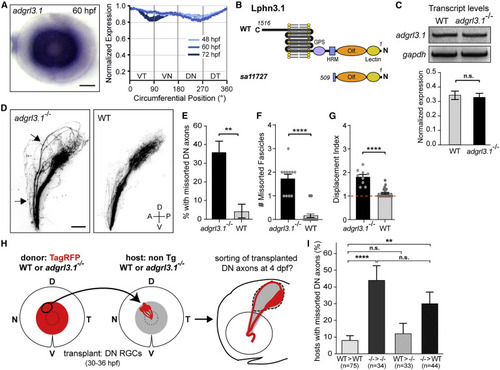
Lphn3.1 signals cell autonomously for pruning missorted DN axons (A) adgrl3.1 ISH staining is detected throughout the RGC layer in the retina throughout development. Scale bar: 50 μm. (B) Schematic of Lphn3.1 protein structure in WT and adgrl3.1sa11727 mutants. Olf, olfactomedin domain; HRM, hormone receptor motif; GPS, GPCR proteolysis site. (C) Analysis of adgrl3.1 transcript levels by RT-PCR in WT and adgrl3.1 mutants at 4 dpf. Unpaired two-tailed t test, p = 0.71. Bars: standard errors. (D) DN axons missort along the dorsal branch of the optic tract in adgrl3.1 mutants (arrows) but not in WT siblings at 4 dpf. Scale bar: 50 μm. (E) Percentage of larvae with missorted DN axons at 4 dpf. One-sided chi-squared test with Yates’s correction, X2 (1, N = 50) = 6.125, p = 0.0067. (F) Number of missorted DN axon fascicles in mutant larvae with sorting defects. Unpaired one-tailed t test, p ˂ 0.0001. (G) Quantification of the displacement index in mutant larvae with sorting defects. Unpaired one-tailed t test, p ˂ 0.0001. n = 9 mutants. (H) DN RGCs of an isl2b:TagRFP donor embryo (WT or adgrl3.1 mutant) were topographically transplanted between 30 and 36 hpf into the DN retina of a WT or adgrl3.1 mutant host. Pre-target sorting of transplanted donor DN axons was assessed at 4 dpf. (I) Quantifications of the percentage of transplanted hosts with missorted donor DN axons. Chi-squared test, X2 (3, N = 186) = 22.51, p ˂ 0.0001. ∗∗p = 0.0023; ∗∗∗∗p ˂ 0.0001. Error bars: standard errors. See also Figure S7. |