- Title
-
The midbody component Prc1-like is required for microtubule reorganization during cytokinesis and dorsal determinant segregation in the early zebrafish embryo
- Authors
- Nair, S., Welch, E.L., Moravec, C.E., Trevena, R.L., Hansen, C.L., Pelegri, F.
- Source
- Full text @ Development
|
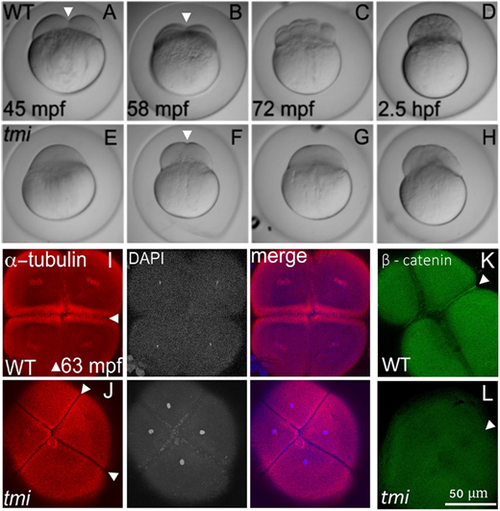
tmi mutant embryos exhibit defects in cytokinesis. (A-H) Developmental time course of wild type (WT; A-D) and tmi mutant (E-H) embryos. In wild type, furrow formation (arrowheads in A,B) and cell division proceeds to generate a blastula. In tmi mutants, incipient furrows form (arrowhead in F) but do not fully contract during cell division, resulting in acellular embryos. (I-L) Immunolabeling of fixed embryos. (I) In wild type, labeling of microtubules (α-tubulin) and DNA (DAPI) show formation of furrows (arrowheads), including reorganization of the microtubular apparatus characteristic of maturing furrows (the FMA, arrowheads in I) as well as nuclear division. (J) In tmi mutants, furrows appear to initiate development as assessed by zones of microtubule exclusion (arrowheads), although the FMA does not fully form, exhibiting a reduction in microtubule enrichment. (K,L) Wild-type embryos (K) show accumulation of β-catenin in mature furrows (arrowhead), but tmi embryos (L) lack β-catenin accumulation (arrowhead). All phenotypes are 100% penetrant with 7-15 embryos per condition in I-L (see Materials and Methods). In spite of acellular phenotype, tmi mutant embryos appear fertilized, judged by the presence of sperm-dependent centrosomes (see Fig. 3 and Dekens et al., 2003; Lindeman and Pelegri, 2012; Yabe et al., 2007) and incipient furrows dividing cells into stereotypically placed, equally sized masses (F,J). |
|
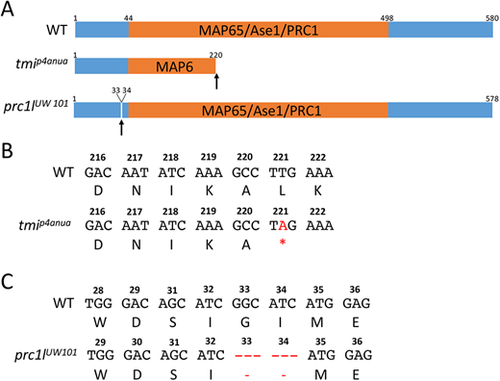
Wild-type and mutant zebrafish Prc1l. (A) Top: Diagram of the predicted wild-type protein, depicting the conserved MAP65/Ase1/PRC1 domain. Middle: The lesion in the ENU-induced maternal-effect allele tmip4anua results in a truncation of the protein deleting a majority of this domain. Bottom: The CRISPR-Cas9-induced allele prc1lUW101 results in the deletion of two conserved amino acids (see Fig. S1B and Fig. S10) N-terminal to the conserved MAP65/Ase1/PRC1 domain. (B,C) Nucleotide sequence and translated amino acids showing the creation of a premature stop codon in tmip4anua (B) and the two-amino acid deletion in prc1lUW101 (C). |
|
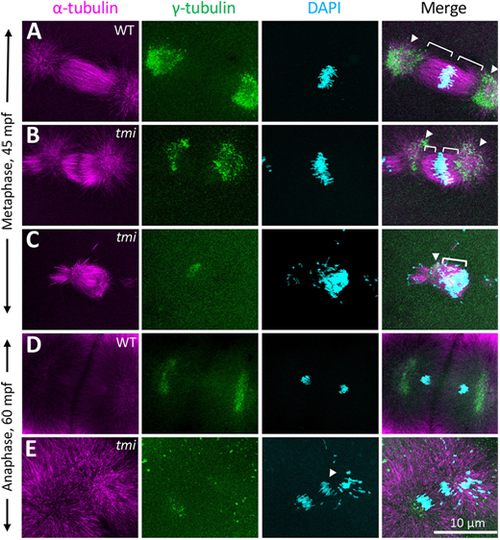
Spindle formation defects in the early mitotic divisions in tmi mutants. (A-C) At metaphase, wild-type asters are highly symmetric (A). In tmi mutants, spindles are often asymmetric (B) or unipolar with chromosomes clustering at one end of the structure (C). Brackets and arrowheads highlight kinetochore microtubule and centrosomes, respectively. (D,E) During anaphase, tmi mutants exhibit chromosomes that fail to segregate (E, arrowhead), which was not observed in wild type (D). Analysis is from embryos at the two- to eight-cell stages (0.751-0.25 hpf), with the specific cell cycle stage assigned by chromosome and spindle morphology. |
|
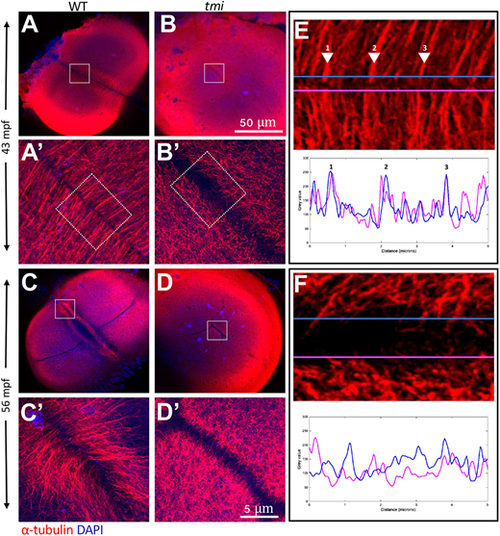
Aberrant reorganization and clustering in the furrow microtubule array. Immunolabeling of fixed embryos to detect microtubules. (A,B) During early stages of furrow formation, the FMA forms along the length of the furrow, consisting of tubule bundles parallel to each other and perpendicular to the furrow (A,A′). tmi mutants fail to show a clearly organized FMA and exhibit a reduction in bundling (B,B′; see also Fig. S5). (C-D′) Upon furrow maturation, tubules of the FMA reorganize, becoming enriched to the distal ends of the furrow and acquiring a tilted angle pointing distally (C,C′), whereas in tmi mutants microtubules fail to reorganize in this manner (D,D′). (E,F) Line scans of FMA bundles along both sides of the furrow show a strong spatial concordance of microtubule bundles in wild type (E, representing the boxed area in A′; numbers correspond to major sites of bundling), consistent with interconnections between bundles across the furrow. Such spatial concordance is absent in tmi mutants (F, corresponding to the boxed area in B′). Images are representative of nine ROIs, each spanning the length of the furrow for each condition (see Fig. S5 and Materials and Methods). Boxed areas in A-D are shown at higher magnification in A′-D′, respectively. |
|
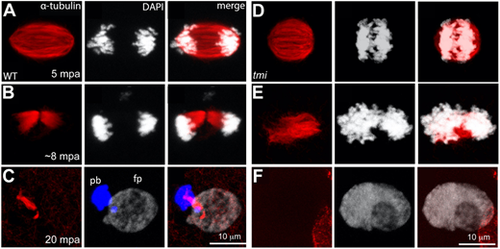
Meiotic spindle defects in tmi mutants. (A-C) In wild-type eggs, spindles for the second meiotic division exhibit an elongated shape (A) that resolves into midbody-like structures (B) and becomes highly bundled during abscission (C). During the last stages of cytokinesis, the midbody becomes asymmetric with a longer region adjacent to the polar body (pb; left DNA mass in C, which becomes condensed), and a shorter region adjacent to the female pronucleus (fp; right DNA mass in C, which undergoes decondensation). (D-F) In eggs from tmi mutant females, spindles appear shorter with less-defined poles (D) and fail to form a well-defined midbody (E; see also Fig. 7B). Subsequently, a single DNA mass is observed (F), likely after failure of cytokinesis for meiosis II followed by fusion. In all panels, DNA is represented in white, except the polar body in C, which has been color-coded in blue in the appropriate z-stack sections to better highlight this structure. |
|
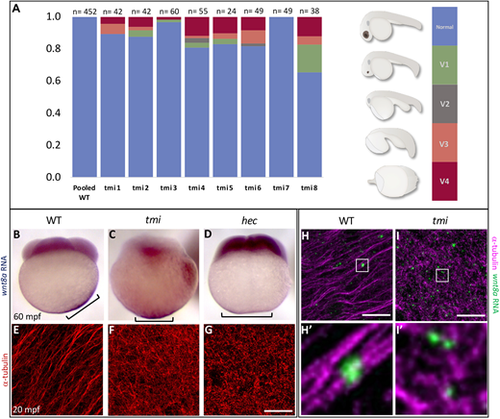
tmi mutants exhibit defects in the segregation of dorsal determinants. (A) Females heterozygous for the maternal-effect tmi mutation consistently produce a fraction of embryos with ventralization phenotypes in 24 h embryos (V1-V4; ventralization series as in Kishimoto et al., 1997), with V4 the most severe, radially symmetric phenotype (see Fig. S7 for live images). (B-D) wnt8a RNA, originally localized to the vegetal pole of the egg, undergoes a lateral shift towards the future dorsal axis in wild-type embryos (B), a behavior absent in embryos from tmi (C) and hec (D) homozygous mutant mothers. Brackets indicate observed extent of wnt8a RNA at the cortex. (E-G) Microtubules at the vegetal cortex develop into a parallel array of microtubule bundles aligned toward the future dorsal axis (E) (Jesuthasan and Strähle, 1997; Tran et al., 2012; Ge et al., 2014). Vegetal microtubule bundling and alignment is defective in embryos from females homozygous mutant for tmi (F) and hecate (G). (H,I) Labeling of wnt8a RNA-containing particles and microtubules at the vegetal cortex of 20 mpf embryos, showing wnt8a RNA localizing to microtubules in both wild type and tmi mutants, despite different microtubule reorganization. H and I are 2D maximum projections of confocal z-stacks (see Movies 1 and 2 to view individual z-slices), and H′ and I′ show magnified views from individual z-slices. Quantification of individual z-slices (see Materials and Methods; Fig. S8) shows no statistically significant difference in the percentage of wnt8a RNA particles that co-occur with microtubules (wild type: 94.4%, n=10 ROIs, 5 embryos; tmi: 93.0%; n=10 ROIs; 5 embryos; P=0.472; unpaired Student's t-test). |
|
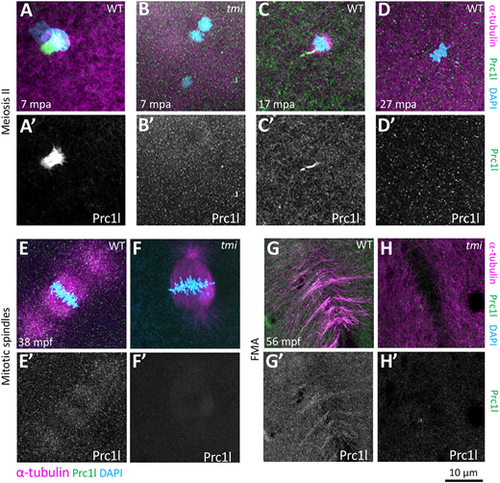
Localization of Prc1l protein to microtubule structures involved in cytokinesis at the animal pole. (A-D′) Prc1l protein localizes to bundling midbody microtubules during meiosis II, (A,A′,C,C′) and becomes delocalized after polar body extrusion (D,D′). tmi mutants do not show antibody labeling (B,B′), as expected from antibody specificity (see also G,H and Fig. S9A). (E-H′) During embryonic mitotic divisions, Prc1l protein localizes to the microtubule apparatus (E,E′) and the FMA (G,G′), with the expected absence of label in tmi mutant embryos (F,F′,H,H′). |
|
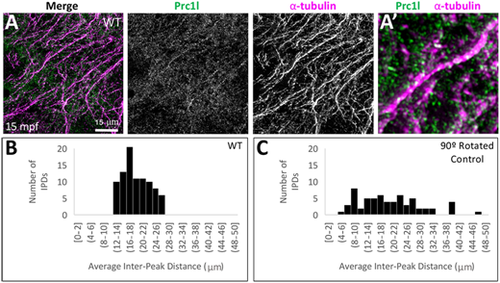
Localization of Prc1l protein to aligned vegetal microtubule arrays at the vegetal pole. (A,A′) Array of aligned bundles of vegetal cortical microtubules and localization of Prc1l protein along bundles in wild type (A, magnification in A′). (B,C) Distribution of distances between sites of Prc1l localization along microtubule tracks, showing a restricted distribution range in wild type (B) and random distribution in a rotated-channel control in wild type (C). The difference between distributions is statistically significant according to a Kolmogorov–Smirnov test (P=0.002; see Fig. S9B,C). |
|
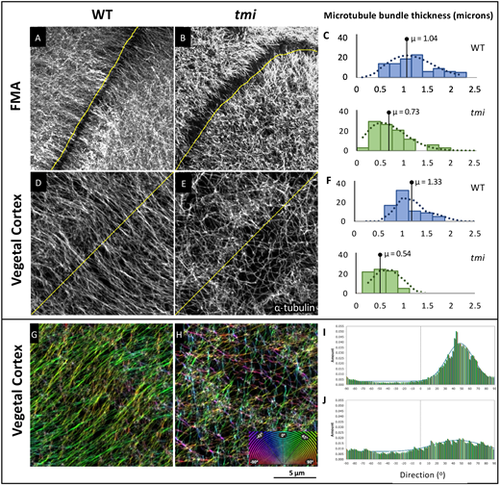
Prc1l functions in microtubule bundling and orientation. (A-C) FMA microtubules. (A) In wild type during furrow formation, microtubules coalesce in thick bundles along the FMA. (B) Microtubules along the FMA in tmi mutant embryos exhibit lack of bundling. (C) Quantification of the average width of the microtubules and microtubule bundles along the FMA in wild-type (blue, n=30) and mutant (green, n=30) embryos at the four-cell stage (56-63 mpf). (D-F) Vegetal cortex microtubules. (D) Microtubule bundles at the vegetal cortex of wild-type embryos. (E) Microtubules at the vegetal cortex of tmi mutant embryos show reduced bundling. (F) Quantification of the average width of vegetal cortex microtubules and microtubule bundles in wild type (blue, n=30) and mutants (green, n=30) at 20 mpf, showing decreased thickness in mutants. Statistical analysis in C,F was carried out using the Mann–Whitney test. (G-J) Microtubule track directionality analysis of vegetal cortex microtubules (RGB 180° spectral color coding). Microtubule tracks exhibit a cohesive orientation in wild type (single-color coding in G, n=6), and a disorientated network in tmi embryos (multi-color coding in H, n=6). Microtubule track angle distribution at 20 mpf (shown in I,J for representative single ROIs) shows regularity in wild type (I) but not mutants (J). The difference between distributions is statistically significant according to a Kolmogorov–Smirnov test (P<0.009). |