- Title
-
Hmx3a Has Essential Functions in Zebrafish Spinal Cord, Ear and Lateral Line Development
- Authors
- England, S.J., Cerda, G.A., Kowalchuk, A., Sorice, T., Grieb, G., Lewis, K.E.
- Source
- Full text @ Genetics
|
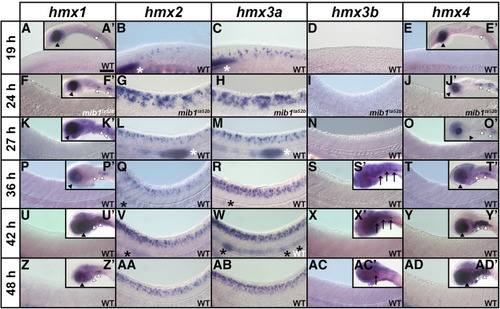
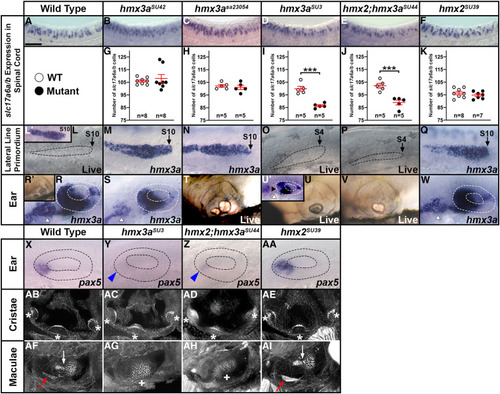
Expression of EXPRESSION / LABELING:
|
|
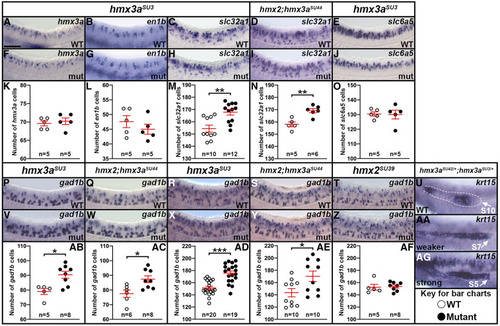
EXPRESSION / LABELING:
|
|
EXPRESSION / LABELING:
PHENOTYPE:
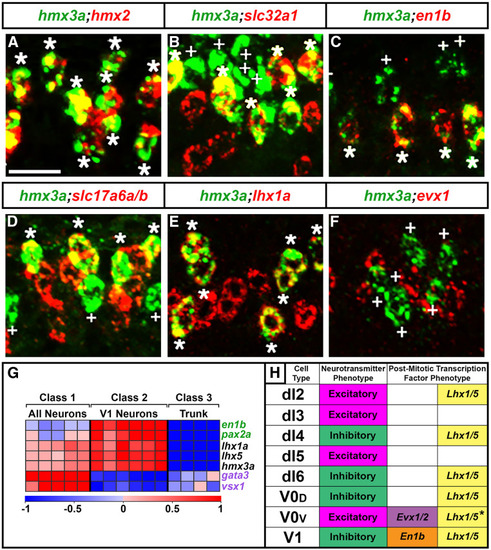
|
|
Summary of |
|
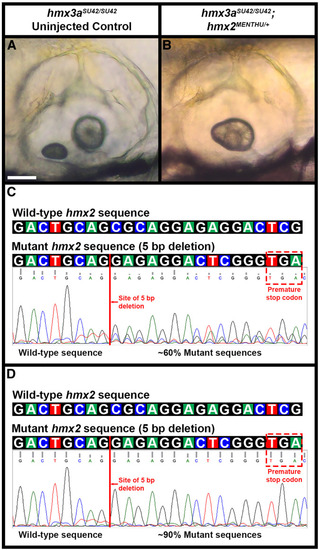
Only some |
|
Analysis of |
|
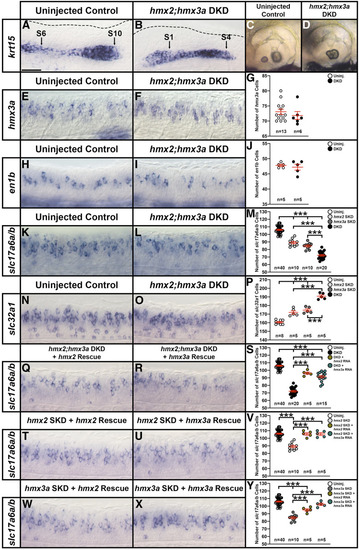
Phenotypic and genotypic analysis of embryos from an incross of PHENOTYPE:
|
|
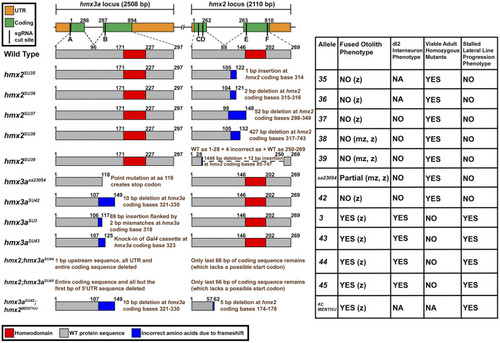
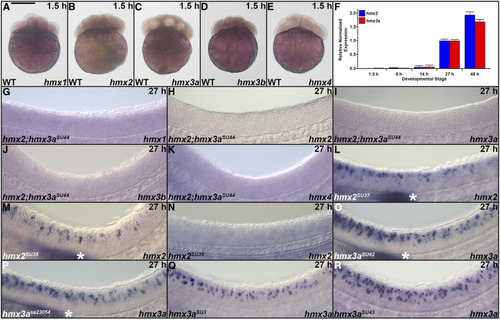
Expression of |