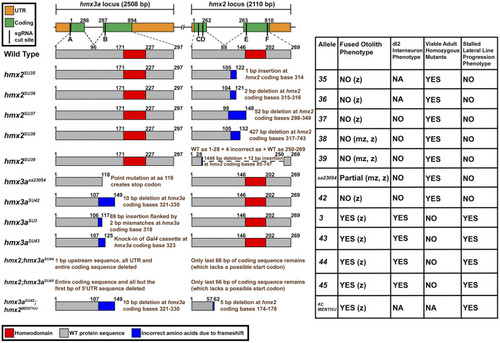
Summary of hmx2/3a mutant alleles analyzed and their phenotypes when homozygous. Left: schematics of 11 mutant alleles and one double mutant analyzed. Top row indicates genomic locus; lower rows indicate predicted protein products. There is a 6454 bp gap between hmx2 and hmx3a. Vertical black bars on genomic locus indicate locations of sgRNA sequences, A–F, used to generate the mutants shown. These sequences and the combinations of sgRNAs used to generate the mutants shown here are listed in Table S2. For each mutant allele, the genomic location plus the nature of the mutation or indel size is shown in brown text at the right side of each mutant protein schematic. Coding bases refer to the translated sequence, e.g., coding bases one to three correspond to the bases encoding the start methionine. Right: Column 1 indicates allele number. Column 2 indicates whether embryos with fused otoliths were observed in incrosses of heterozygous (z) or homozygous (mz) parents (also see Table 3 and Figure 5). Column 3 indicates whether a reduction in the number of spinal excitatory cells was observed at 27 hpf in homozygous mutants, as assayed by in situ hybridization for slc17a6a/b (Figure 5 and data not shown). Column 4 indicates whether viable adult homozygous mutants were recovered. In all cases where adult homozygous mutants were identified, the numbers of these fish are not statistically significantly different (P > 0.214) from expected Mendelian ratios, as assayed by a chi-squared test. P-values are provided in Table 3. Column 5 indicates whether embryos with stalled lateral line progression phenotypes were observed in incrosses of heterozygous parents. All hmx2 stable mutant alleles recovered to date are homozygous viable and homozygous mutants do not have a reduction in the number of glutamatergic spinal cord interneurons or otolith or stalled lateral line progression phenotypes. This is the case, even for embryos from incrosses of fish homozygous mutant for the most severely deleted hmx2 alleles (hmx2SU38 and hmx2SU39). hmx3asa23054 mutants are also homozygous viable. They have variable, incompletely penetrant, otolith fusion phenotypes. Strikingly, only 27.41% of embryos from an incross of homozygous mutant parents have otolith fusion phenotypes (Table 3). hmx3asa23054 mutants do not have a reduction in the number of glutamatergic spinal cord interneurons or stalled lateral line progression phenotypes. hmx3aSU42 mutants are homozygous viable and do not have any obvious abnormal phenotypes, even though this allele should encode a protein with the same number of WT amino acids as hmx3aSU43 and only one more WT amino acid than hmx3aSU3. hmx2;hmx3aSU44 and hmx2;hmx3aSU45 differ only in the amount of upstream sequence that is deleted and have identical phenotypes.
|

