- Title
-
A quantitative modelling approach to zebrafish pigment pattern formation
- Authors
- Owen, J.P., Kelsh, R.N., Yates, C.A.
- Source
- Full text @ Elife
|
( |
|
( |
|
( |
|
( |
|
( |
|
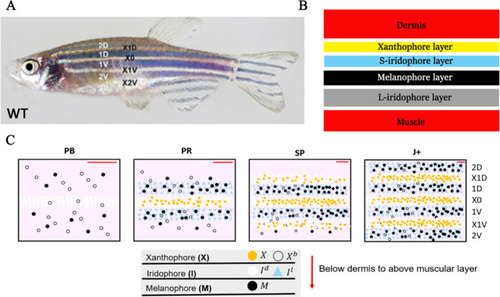
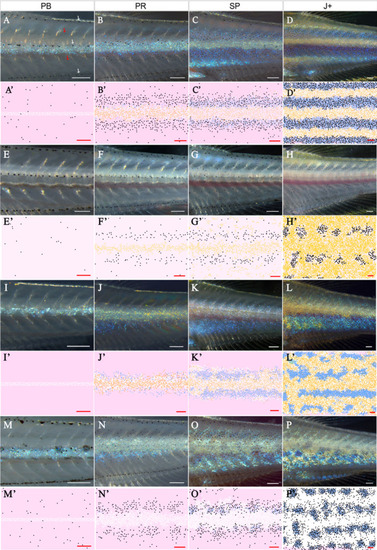
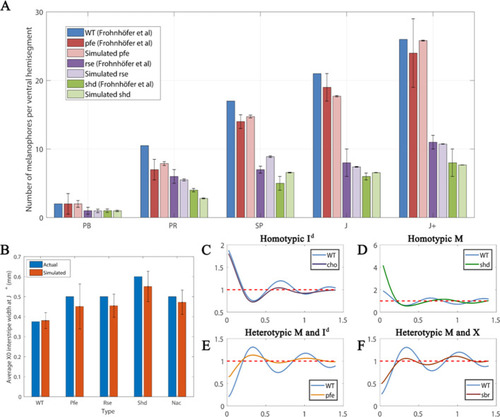
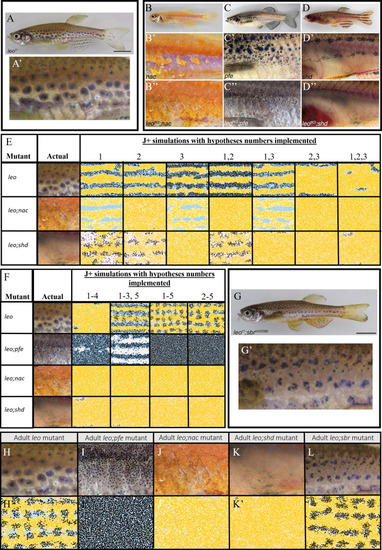
Each square is an example WT simulation at stage J+ where each rate parameter is perturbed to 1+ |
|
( |
|
( |
|
( |
|
( |
|
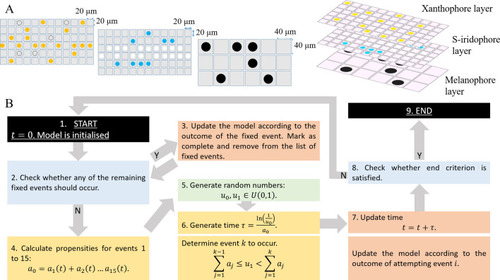
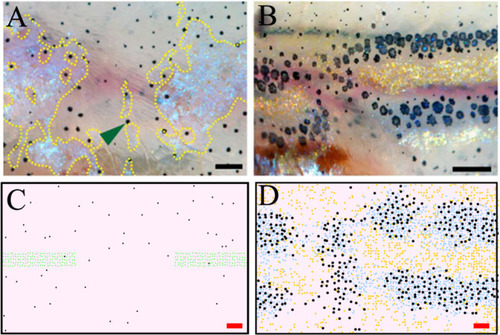
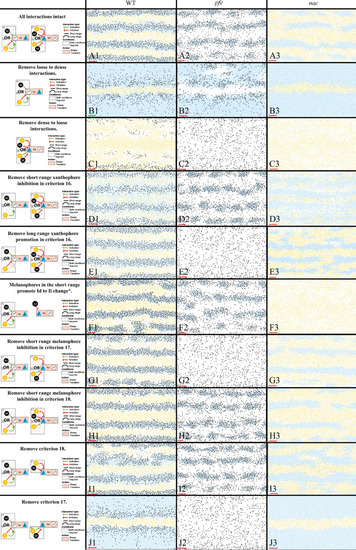
The first column displays a diagram of the S-iridophore interactions which remain (all other cell-cell interactions are unchanged). Columns 2–4 are representative simulations of WT, |
|
( |
|
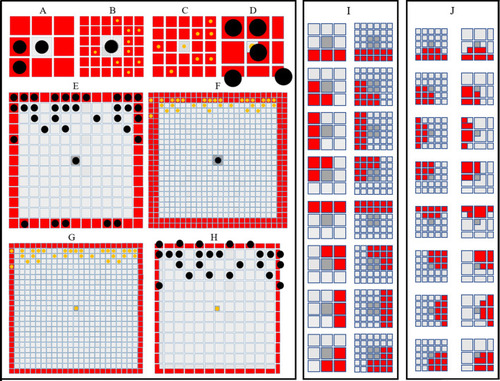
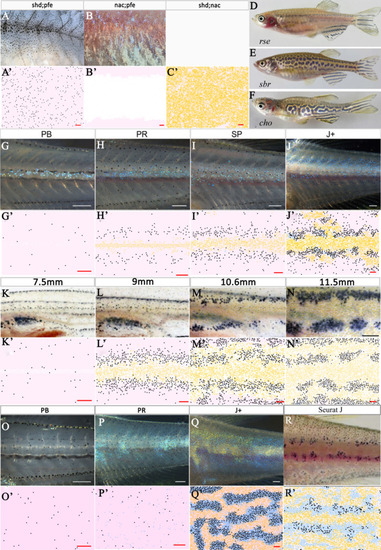
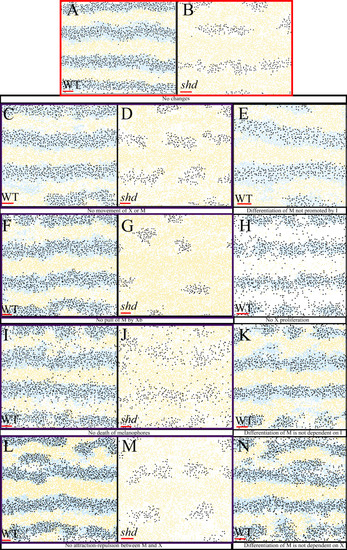
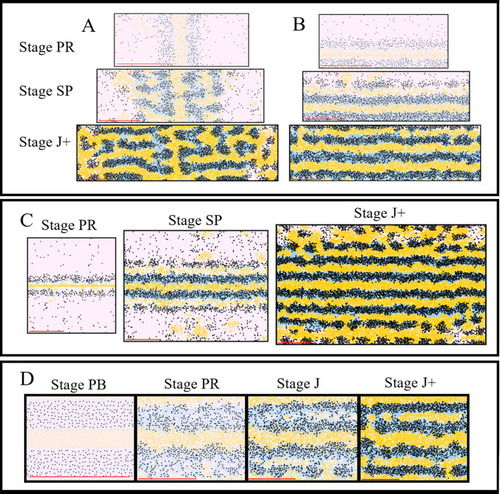
The simulated domains at stages PR, SP and J+ wherein the following are changed ( |
|
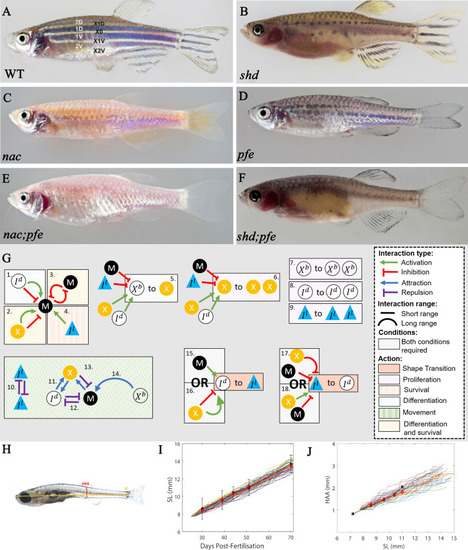
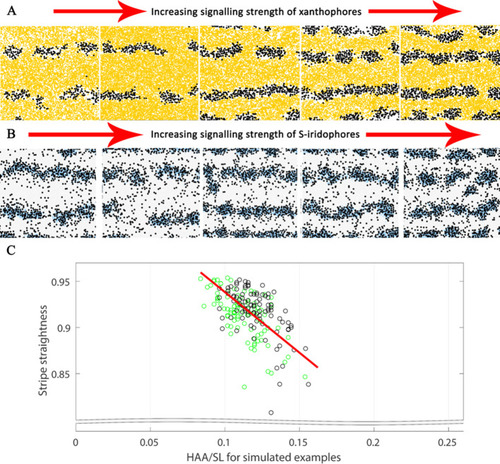
The effects of different signalling strengths in |
|
( |