- Title
-
Zebrafish Prion Protein PrP2 Controls Collective Migration Process during Lateral Line Sensory System Development
- Authors
- Huc-Brandt, S., Hieu, N., Imberdis, T., Cubedo, N., Silhol, M., Leighton, P.L., Domaschke, T., Allison, W.T., Perrier, V., Rossel, M.
- Source
- Full text @ PLoS One
|
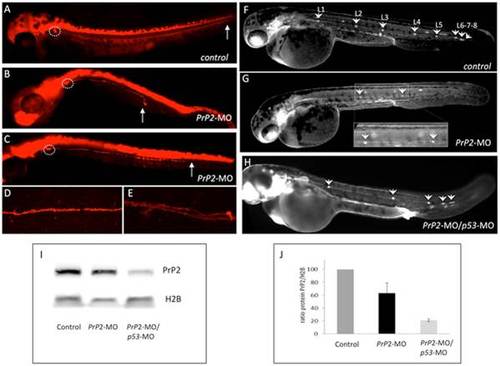
PrP2 decrease expression results in abnormal PLL development. A. In control embryos at 48 hours post fertilization (hpf) from nbt-dsred line that labels neurons and axons, the PLL nerve develops from the PLL ganglia until the tip of the tail (arrow). B–C. In PrP2-MO embryos, a range of defects for the PLL nerve is observed with premature arrest (B, C). D. Control PLL nerve fibers are tightly while PrP2-MO fibers exhibit abnormal branches were observed in severe cases (E). F. In control claudinB-GFP embryos at 48 hpf all derivatives issuing from the primordium express GFP and appear normal. Five regularly spaced neuromasts are present, with 3 terminal neuromasts at the tip of the tail. Due to embryo transparency, the neuromasts on the other side of the embryo are also visible. In PrP2-MO (G) and PrP2-MO/p53-MO (H) injected embryos (in order to avoid off-target defects), neuromast numbers are reduced, and often irregularly spaced. I–J. Western blot analysis of PrP2 expression using SAF84 antibody shows a decrease of the PrP2 protein after morpholino injections (n = 3 independent experiments). |
|
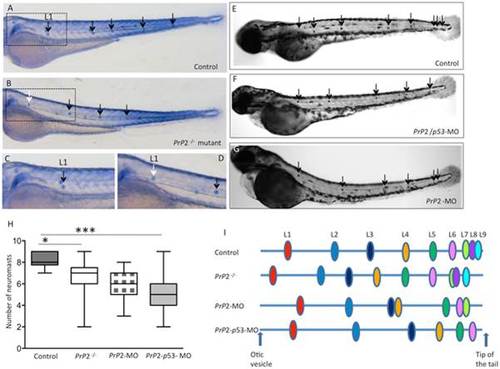
PrP2 is involved in PLL development. A, C. Alkaline phosphatase staining of the trunk neuromasts (arrows) in wild type embryos at 72 hpf. C. High magnification of boxed area in A. B, D. In Prp2/ mutant, less neuromasts are visible with the first neuromast displaced anteriorly (B, white arrow) D. High magnification of boxed area in B. E–G. In Control (E), PrP2-MO/p53-MO (F) and PrP2-MO (G), the total neuromast number is decreased in morphants. H. Quantification of total neuromast number shows a significant decrease in morphants and mutants compared to control. I. Neuromast position along the somite axis, scheme adapted from [28]. In Prp2/ mutant, L1 is retrieved anteriorly to the normal L1 position while in morphants, the present neuromasts are displaced posteriorly. *: p<0.05, ***: p<0.001. PHENOTYPE:
|
|
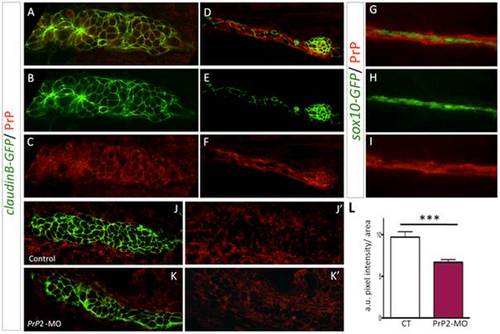
PrP2 is expressed in the primordium. A–C. Immunofluorescence on 30 hpf claudinB-GFP embryos using anti-PrPC antibodies, revealed PrP expression at the membrane of primordium cells during the migration process. D–F. Neuromast cells as well as interneuromastic cells are positive for PrP2. What is represented by the arrows here? G–I. PrP immunofluorescence on 48 hpf sox10-GFP embryos, showed that Schwann cells are included in PrP expression area. J, J2. Expression level of PrP2 within the primordium in control embryos. K, K2. Expression level of PrP2 within the primordium in Prp2-MO. L. Quantification analysis using pixel intensity measurement with ImageJ software, within the primordium areas, reveals a significant decrease of PrP2 expression level in Prp2-MO. a.u.: arbitrary unit of pixel intensity, area: primordium anlage (*** p<0.001, Mann-Whitney test, n = 10). |
|
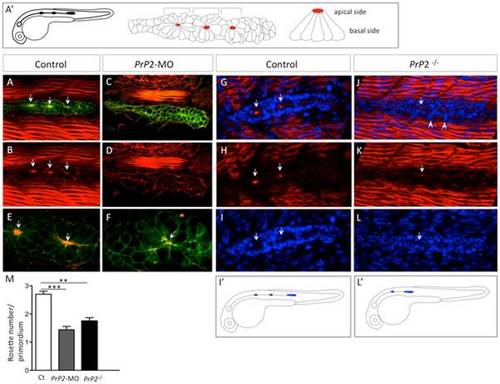
Primodium disorganisation and absence of rosette formation. A′. Schematic representation of a normal claudinB-GFP embryo at 30 hpf and detailed organization of the primordium with rosette structure. Red spot indicates a normal concentration point of actin. A, B. Phalloidin staining (Phalloidin-TRITC) in control embryo claudinB-GFP, at 30 hpf, is observed in muscle cells and within the primodium at the center of the rosette (arrows), on the apical side. C, D. Phalloidin-TRITC staining in PrP2-MO, no rosette structure is observed and no actin concentration is found. E, F. Higher magnification shows the co-localization of central actin concentration with claudinB-GFP at the rosette center in control. In morphants, cell disorganization is observed and no actin concentration is observed associated with the absence of a rosette. G–I. Phalloidin staining and DAPI nuclei labeling highlight the primordium and rosette center (arrows) in control embryos. J–L. In PrP2/ mutants, actin apical localization in rosette was severely reduced or barely detectable (arrow) and primordium organization at the periphery was impaired: loose cells were visible on the border (arrowheads). I2, L2. In PrP2/ mutant, the primordium position was often delayed and the first neuromast deposited close to the ear. M. Quantification of rosette number was established in control (n = 20), PrP2-MO (n = 84) and PrP2/ mutant (n = 28) using actin staining at the center, **: p<0.01, ***: p<0.001, Student t test. See also associated Movies S1–S5. |
|
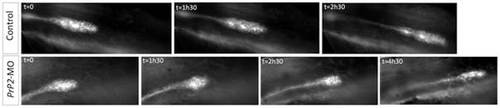
Loss of cell-cell contact and disorganization of collective migration of the primordium. Select time points from time-lapse recording of primordium migration in claudinB-GFP embryos. In control embryos, primordium migration is continuous, with a neuromast deposition (time point 2h30) and out of view at the time point 4h30 (not shown). In contrast in one PrP2 morphant example, representative of the observed phenotypes, primordium migration shows a progressive rounded shape and arrest. See also Movies S6 and S7. |
|
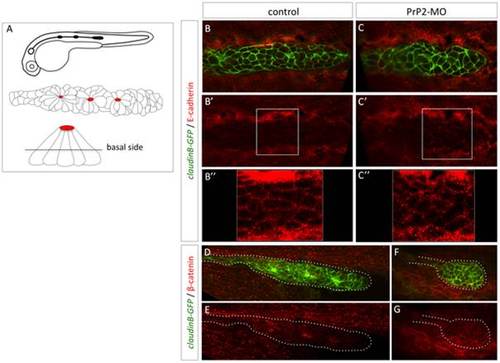
Delocalisation of E-cadherin and beta-catenin in primordium cells in absence of PrP2. A. Schematic representation of claudinB-GFP embryo at 30 hpf, primordium structure, and transversal view of a rosette showing the level of confocal focus plan. B–B′′. In control embryos at 30 hpf, E-cadherin is expressed at the membrane level at the basal side of the primordium. C–C′′. In morphant embryos, E-cadherin is observed in the cytoplasm and cellular membrane at basal and apical levels. The staining pattern is more punctuated in morphant compared to control. D, E. In control embryos, beta catenin expression is observed at the membrane level and at the center of rosette structures. F, G. In morphant, small rounded primordium shows a membrane homogeneous pattern with no rosette. |
|
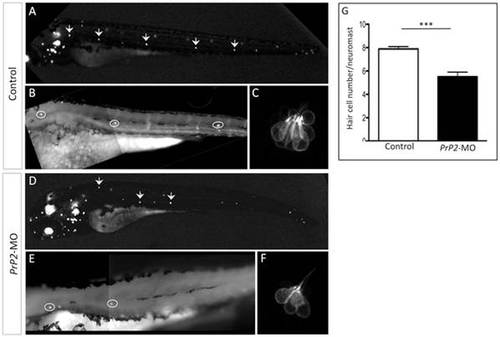
Decreased hair cell number of PLL neuromast in PrP2-MO. A–C. In control embryos at 48 hpf, Brn3-GFP fluorescence labels hair cells of neuromasts and ear. High magnification of the first 3 neuromasts and zoom of the first neuromast show 8 hair cells. D–F. Morphants displays reduced number of neuromasts. High magnification shows smaller hair cells number. G. Quantification indicates significant reduction of hair cells/neuromast, independently of the neuromast position (mean hair cell number: 5.5±0.4, n = 36, compare to control 7.9±0.2, n = 20, p<0.001). |
|
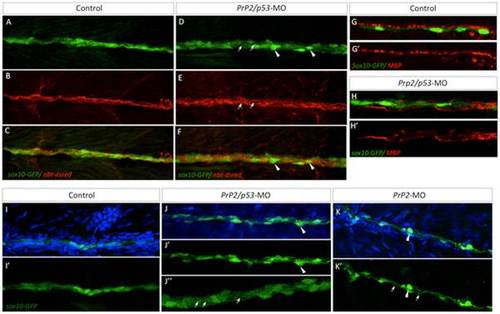
Loss of PLL nerve fasciculation and associated myelination in absence of PrP2. A–C. In control larvae at 5 dpf, sox10-GFP cells are tightly organized along the PLL nerve (nbt-dsred). D–F. Double morphant PrP2/p53 displayed an enlarged PLL nerve (with defasciculated axons, arrows) and rounded Schwann cells (arrowheads). G–G′. In 5 dpf larvae MBP labeling is observed in close apposition to sox10-GFP cells and formed a homogeneous line. H–H′. MBP labeling is altered and partially missing while Schwann cells are disorganized. I–I′. At 7 dpf, control larvae display tightly and regularly organized sox10-GFP positive cells. J–K′. In PrP2/p53 morphants (J–J′′), loosened Schwann cell processes are observed (arrows) as well as rounded cells (arrowheads) in PrP2 morphants (K–K′). Results obtained from five independent experiments (n = 160 embryos). |