- Title
-
Expression and functional characterization of Smyd1a in myofibril organization of skeletal muscles
- Authors
- Gao, J., Li, J., Li, B.J., Yagil, E., Zhang, J., and Du, S.J.
- Source
- Full text @ PLoS One
|
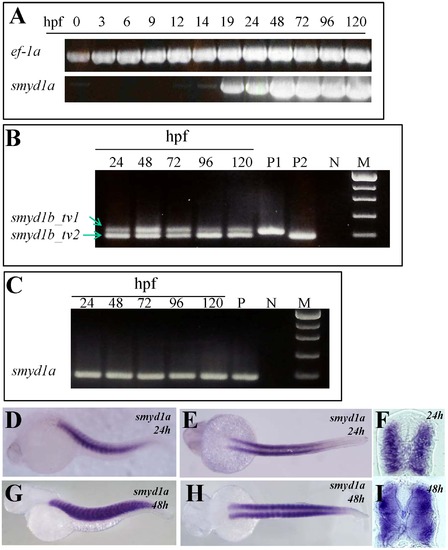
The temporal and spatial pattern of smyd1a expression in zebrafish embryos. A. RT-PCR results show temporal expression of smyd1a in zebrafish embryos from fertilization to day 5. Elongation factor 1-alpha (ef-1α) was used as control. B. RT-PCR analysis shows the alternative splicing of smyd1b exon 5 generating two isoforms of smyd1b, smyd1b_tv1 and smyd1b_tv2. C. RT-PCR analysis shows the lack of alternative splicing of exon 5 in smyd1a in zebrafish embryos. D–I. Whole mount in situ hybridization shows the spatial pattern of smyd1a mRNA expression using a dig-labeled antisense probe. smyd1a expression was detected in skeletal muscles of zebrafish embryos at 24 (D, E, F) and 48 (G, H. I) hpf. D, G represent the side view; E, H repre4sent the dorsal view; F, I represent the cross sections. |
|
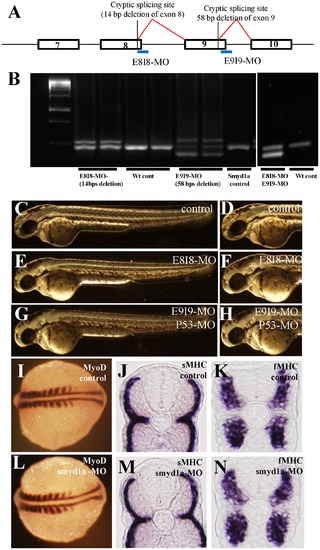
Knockdown of smyd1a splicing by E8I8-MO and E9I9-MO. A. Location of the E8I8-MO and E9I9-MO splicing blockers at the junction of exon8/intron8 and exon 9/intron 9, respectively. Defective splicing using a cryptic splicing site in exon 8 in E8I8-MO injected embryos resulted in a deletion of 14. Defective splicing using a cryptic splicing site in exon 9 in E9I9-MO injected embryos resulted in a deletion of 58 bp. B. RT-PCR showing the defective splicing induced by the E8I8-MO or E9I9-MO or both MOs. Compared with the PCR results from the wild type (wt) control embryos where a single band was generated, two bands were observed in the E8I8-MO injected embryos. Sequence analysis revealed that the smaller band contained a 14 bp deletion at the end of exon 8. The upper band represented the heteroduplex formed by the normal and defectively spliced products. Similarly, three bands were detected in E9I9-MO injected embryos. Sequence analysis revealed that the smaller band contained a 58 bp deletion at the end of exon 9. The middle band was the normal spliced product, whereas the upper band represented the heteroduplex formed by the normal and defectively spliced products. Two major bands were detected in E8I8-MO and E9I9-MO co-injected embryos. Sequence analysis revealed that the smaller band resulted from defective splicing. C–H. Morphology of smyd1a knockdown embryos at 48 hpf. Zebrafish embryos injected with control MO (C, D), E8I8-MO (E, F) or E9I9-MO (G, H) at 48 hpf. I–N. Knockdown of smyd1a expression had no effect on myoblast specification and early differentiation of slow and fast myofibers. In situ hybridization showing normal MyoD expression in control-MO (I) or smyd1a MO (L) injected embryos at 14 hpf. Cross views of slow (J, M) or fast (K, N) MHC expression in control (J, K) or smyd1a MO (M, N) injected embryos at 24 hpf. EXPRESSION / LABELING:
|
|
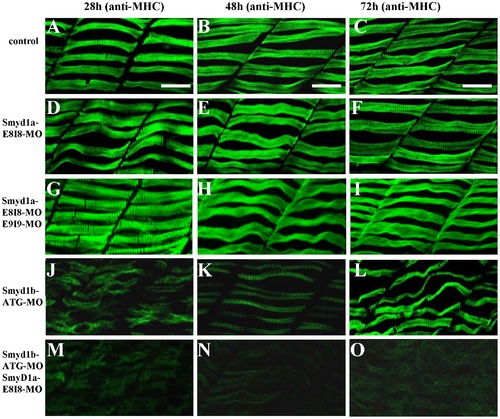
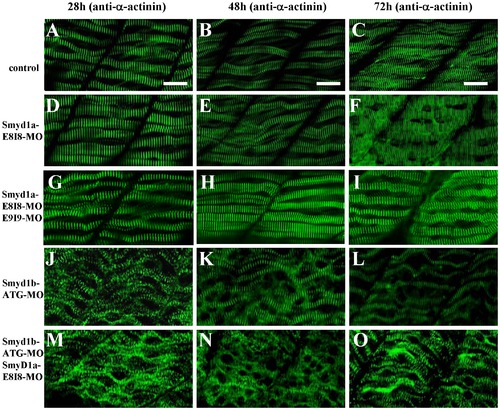
The effect of smyd1a and/or smyd1b knockdown on myosin thick filament organization in skeletal muscles. Zebrafish embryos injected with smyd1b MO or smyd1a MO or both were fixed at 28, 48 and 72 hpf. Myosin thick filament organization was analyzed by immunostaining with the F59 antibody which recognizes the myosin heavy chain (MHC) in slow muscles, and followed by FTIC-labeled secondary antibody. The images represent side view of trunk muscles around segment 10. A–C. Lateral view of thick filament organization in slow muscle fibers of control-MO injected embryos at 28 (A), 48 (B) and 72 (C) hpf. D–F. Lateral view of thick filament organization in slow muscle fibers of smyd1a E8I8-MO injected embryos at 28 (D), 48 (E) and 72 (F) hpf. G–I. Lateral view of thick filament organization in slow muscle fibers of smyd1a E8I8-MO and E9I9-MO co-injected embryos at 28 (G), 48 (H) and 72 (I) hpf. J–L. Lateral view of thick filament organization in slow muscle fibers of smyd1b ATG-MO injected embryos at 28 (J), 48 (K) and 72 (L) hpf. M–O. Lateral view of thick filament organization in slow muscle fibers of smyd1a E8I8-MO and smyd1b ATG-MO co-injected embryos at 28 (M), 48 (N) and 72 (O) hpf. Scale bars: 20 μm in A–C. EXPRESSION / LABELING:
PHENOTYPE:
|
|
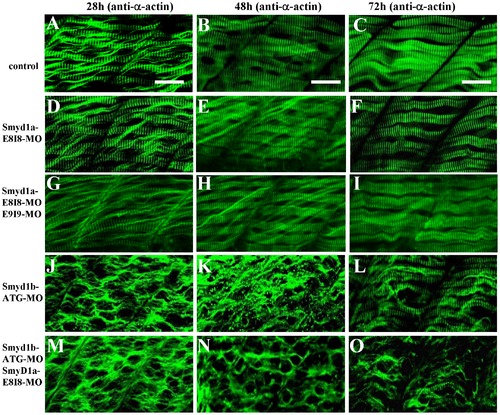
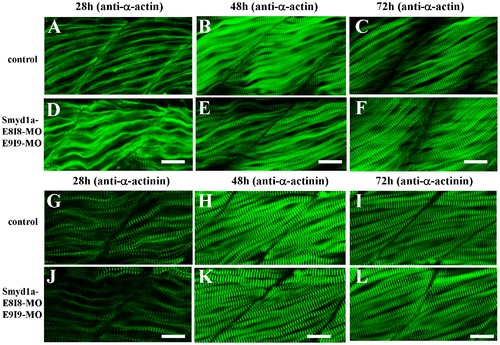
The effect of smyd1a or smyd1b single or double knockdown on thin filament organization in skeletal muscles. Zebrafish embryos injected with smyd1b MO or smyd1a MO or both were fixed at 28, 48 and 72 hpf. α-actin thin filament organization was analyzed by immunostaining with anti-α-actin antibody (Acl-20.4.2), and followed by FTIC-labeled secondary antibody. The images represent side view of trunk muscles around segment 10. A–C. Lateral view of thin filament organization in skeletal muscle fibers of control-MO injected embryos at 28 (A), 48 (B) and 72 (C) hpf. D–F. Lateral view of thin filament organization in skeletal muscle fibers of smyd1a E8I8-MO injected embryos at 28 (D), 48 (E) and 72 (F) hpf. G–I. Lateral view of thin filament organization in slow muscle fibers of smyd1a E8I8-MO and E9I9-MO co-injected embryos at 28 (G), 48 (H) and 72 (I) hpf. J–L. Lateral view of thin filament organization in skeletal muscle fibers of smyd1b ATG-MO injected embryos at 28 (J), 48 (K) and 72 (L) hpf. M–O. Lateral view of thin filament organization in skeletal muscle fibers of smyd1a E8I8-MO and smyd1b ATG-MO co-injected embryos at 28 (M), 48 (N) and 72 (O) hpf. Scale bars: 20 μm in A–C. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The effect of smyd1a or smyd1b single or double knockdown on the Z-line organization in skeletal muscles. Zebrafish embryos injected with smyd1b MO or smyd1a MO or both were fixed at 28, 48 and 72 hpf. Z-line organization was analyzed by immunostaining with anti-α-actinin antibody (EA-53), and followed by FTIC-labeled secondary antibody. The images represent side view of trunk muscles around segment 10. A–C. Lateral view of Z-line organization in skeletal muscle fibers of control-MO injected embryos at 28 (A), 48 (B) and 72 (C) hpf. D–F. Lateral view of Z-line organization in skeletal muscle fibers of smyd1a E8I8-MO injected embryos at 28 (D), 48 (E) and 72 (F) hpf. G–I. Lateral view of Z-line organization in skeletal muscle fibers of smyd1a E8I8-MO and E9I9-MO co-injected embryos at 28 (G), 48 (H) and 72 (I) hpf. J–L. Lateral view of Z-line organization in skeletal muscle fibers of smyd1b ATG-MO injected embryos at 28 (J), 48 (K) and 72 (L) hpf. M–O. Lateral view of Z-line organization in skeletal muscle fibers of smyd1a E8I8-MO and smyd1b ATG-MO co-injected embryos at 28 (M), 48 (N) and 72 (O) hpf. Scale bars: 20 μm in A–C. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The effect of smyd1a knockdown on the sarcomere organization in fast muscles. Zebrafish embryos co-injected with smyd1a E8I8 and E9I9 MOs were fixed at 28, 48 and 72 hpf. The sarcomere organization of thin filaments and Z-lines was analyzed by immunostaining with anti-α-actin and anti-α-actinin antibody, respectively, and followed by FTIC-labeled secondary antibody. The images represent side view of trunk muscles around segment 10. A–C. Lateral view of thin filament organization in skeletal muscle fibers of control-MO injected embryos at 28 (A), 48 (B) and 72 (C) hpf. D–F. Lateral view of thin filament organization in skeletal muscle fibers of smyd1a MO injected embryos at 28 (D), 48 (E) and 72 (F) hpf. G–I. Lateral view of Z-line organization in skeletal muscle fibers of control-MO injected embryos at 28 (G), 48 (H) and 72 (I) hpf. J–L. Lateral view of the Z-line organization in skeletal muscle fibers of smyd1a MO injected embryos at 28 (J), 48 (K) and 72 (L) hpf. Scale bars: 20 μm in D–F and J–L. EXPRESSION / LABELING:
|
|
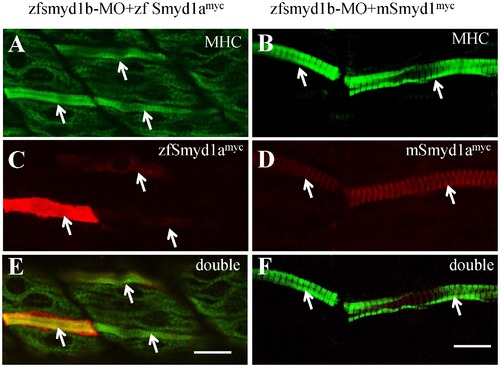
The myofibril defects from smyd1b knockdown could be rescued by ectopic expression of zebrafish smyd1a or mouse Smyd1. The smyd1b ATG-MO was co-injected with smyd1b:zfsmyd1amyc or smyd1b:mSmyd1myc transgene into zebrafish embryos at 1 or 2 cells stages. Myosin thick filament organization and transgene expression were analyzed by double immunostaining with anti-MHC (F59, green) and anti-myc (9E10, red) antibodies. A, C, E. Double immunostaining with anti-MHC (A) and anti-myc (C) antibodies shows the normal thick filament organization in myofibers expressing the myc-tagged zebrafish smyd1a transgene at 24 hpf. E, merged picture of A and C. B, D, F. Double immunostaining with anti-MHC (B) and anti-myc (D) antibodies shows the normal thick filament organization in myofibers expressing the myc-tagged mouse Smyd1 transgene at 24 hpf. F, merged picture of B with D. D and F showed the sarcomeric localization of myc-tagged mouse Smyd1 (red). The myc-tagged mouse Smyd1 was localized in the middle of the MHC thick filament (F), a region normally occupied by the M-line. Scale bars: 20 μm in E; 14 μm in F. |
|
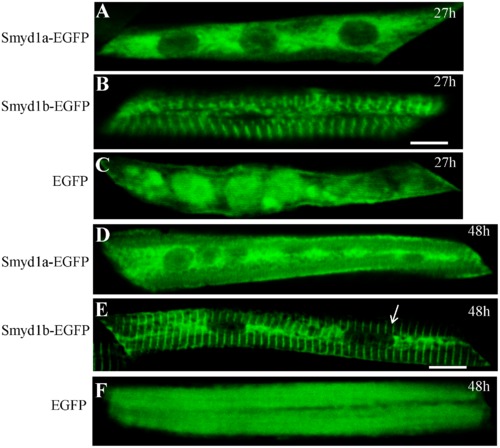
Characterization of the Smyd1a subcellular localization using the Smyd1a-EGFP fusion protein. DNA constructs expressing the zebrafish Smyd1a-EGFP or Smyd1b-EGFP fusion proteins or EGFP control were injected into zebrafish embryos. Their expression and localization were determined in myofibers of the injected zebrafish embryos at 27 (A, B, C) and 48 (D, E, F) hpf. A and D represent Smyd1b-EGFP; B and E represent Smyd1a-EGFP; C and F represent EGFP control. Scale bars: 6 μm in B; 10 μm in E. |