- Title
-
Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved
- Authors
- Soza-Ried, C., Hess, I., Netuschil, N., Schorpp, M., and Boehm, T.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
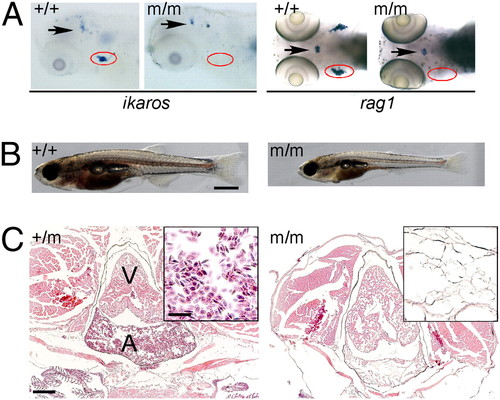
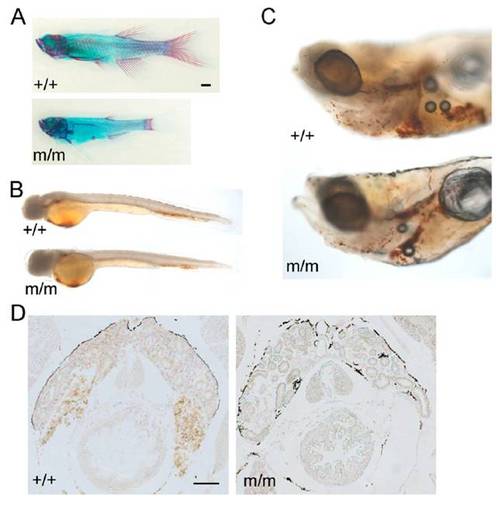
Phenotype of IP109 mutants. (A) Whole-mount RNA in situ hybridization of wild-type (+/+) and homozygous IP109 mutant (-/-) embryos with probes for ikaros (lateral views; Left) and rag1 (dorsal views; Right) at 5 d postfertilization (dpf). Note that ikaros is also expressed in neurons (arrows); this hybridization signal serves as an internal positive control for the hybridization process. In the hybridizations with rag1, a gh probe labels growth hormone-producing cells in the hypophysis (arrows) and serves as control for hybridization. The region of the thymus is encircled. No differences were seen between +/+ and +/m fish. Table S1 has probe details. (B) Macroscopic view of wild-type (Left) and mutant (Right) fish at 20 dpf. Note the smaller size and pale appearance of mutants. (Scale bar: 1 mm.) (C) Histological sections through the regions of the heart (A, atrium; V, ventricle) of heterozygous (Left) and homozygous mutant (Right) fish at 8 wk of age. Note the complete absence of erythrocytes in the mutant fish (Fig. S1). H&E staining. (Scale bars: 400 μm; Inset, 100 μm.) EXPRESSION / LABELING:
PHENOTYPE:
|
|
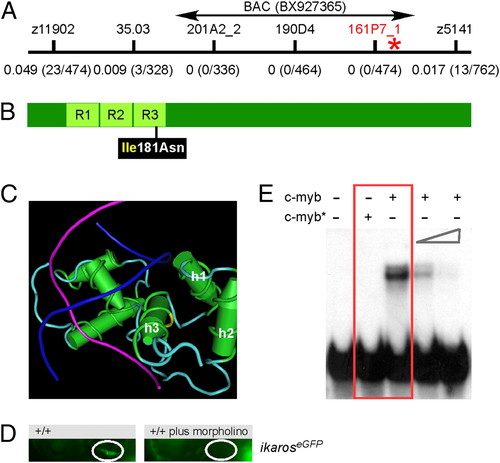
A deleterious missense mutation in the DNA binding domain of zebrafish c-myb. (A) Genetic mapping of the IP109 mutation on chromosome 23. The locations of informative markers are indicated; recombination frequencies are given in brackets below their names (details in Table S3). The position of BAC BX927365 encompassing the c-myb gene that was used for complementation analysis is shown. (B) Schematic indicating the location of the isoleucine (Ile) to asparagine (Asn) missense mutation relative to the three repeat domains (R) of the DNA binding domain in the c-myb protein. (C) The side chain of isoleucine 181 in the third helix (h3) of repeat 3 (yellow area on the carbon trace) points away from the DNA double helix (strands in blue and magenta) to the first (h1) and second (h2) helix of repeat 3. This figure was rendered using Cn3D from PDB ID 1MSE (17). (D) Injection of an antisense c-myb morpholino oligonucleotide into wild-type fish transgenic for an ikaros:eGFP reporter recapitulates the thymic homing defect observed in c-myb mutants. The thymic rudiment is encircled. Lateral views, 4 dpf. (E) The mutant version of c-myb lacks in vitro DNA binding activity. The N-terminal one-half (amino acids 1–318) of the mouse c-myb protein, encompassing the highly conserved DNA binding domain, was expressed in E. coli; the I181N mutation was introduced by site-directed mutagenesis. The mutant protein (c-myb*) does not interact with the radioactively labeled DNA probe containing a c-myb consensus binding site (red box); excess unlabeled binding sites compete with the radiolabeled probe (two right-most lanes). Equal amounts of wild-type and mutant proteins were used (Fig. S2). PHENOTYPE:
|
|
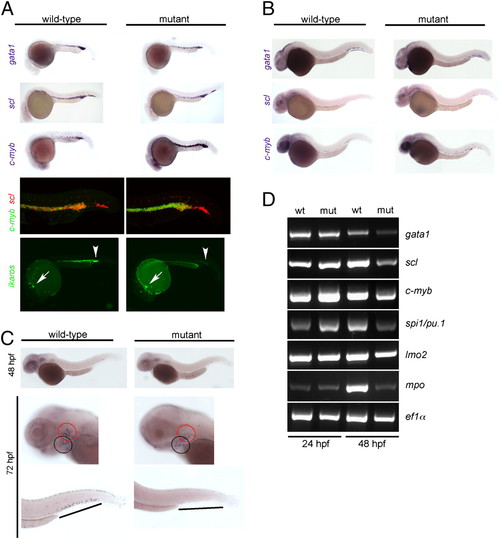
Characterization of primitive hematopoiesis. (A) Whole-mount RNA in situ hybridizations (WISH) were performed at 24 hpf with probes specific for scl (merge composed of three pictures), gata1, and c-myb. Hybridizations using single probes are shown in the top three panels. A double-fluorescent in situ hybridization for c-myb (green fluorescence) and scl (red fluorescence) is shown in the penultimate panel (merge of 22 optical slices at 5-μm thickness for wild-type and 20 slices for mutant embryos; Fig. S3 has additional data). The expression of ikaros was visualized using an ikaros:eGFP transgenic reporter (bottom panel). Note the normal number and fluorescence intensity of embryonic macrophages located in the anterior part of the embryos (arrows) and the diminished signals in the caudal hematopoietic tissue (CHT) and intermediate cell mass (ICM) of mutants (arrowheads); the embryonic macrophages are highly motile cells (Movies S9 and S10). (B) WISH analyses at 36 hpf using the indicated probes. All photographs are composites of three pictures, with the exception of the gata1 hybridization of mutant embryo, which is composed of four pictures. (C) WISH analyses for c-myb expression at the indicated time points. The photographs for the 48-hpf time-point are merged from three pictures. In the images taken at 72 hpf, the pharyngeal arches are marked with a black circle, and the thymic rudiment is marked with a red circle (Middle); the hematopoietic tissue in the tail regions (Bottom) is indicated by lines. (D) Gene-expression analysis in c-myb mutants. RT-PCR was performed at the indicated time points for the indicated genes; elongation factor 1 α (ef1α) serves as a control for cDNA integrity and a standard. EXPRESSION / LABELING:
PHENOTYPE:
|
|
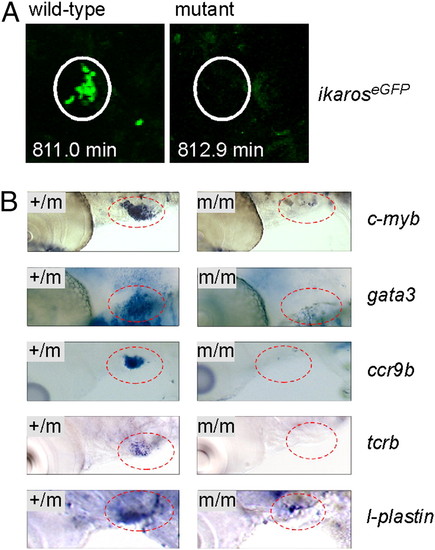
Failure of thymopoiesis in c-myb mutants. (A) Lack of thymus colonization in c-myb mutants transgenic for ikaros:eGFP. Still photographs were taken from Movies S9 and S10 at the indicated time points of the observation period (t0 = 55 hpf), equivalent to <68 hpf. Note the cluster of green cells in the thymus (encircled), whereas the thymus of mutants lacks such cells. A progenitor cell approaching the thymus is seen at the bottom right corner of the image of wild-type fish. (B) No evidence for thymopoiesis in early larvae. WISH was done with probes indicated at 5 dpf; the thymic area (encircled) is shown. Note the presence of small numbers of cells expressing l-plastin in the c-myb mutants, a marker associated with the myelo-monocytic lineage. This most likely represents embryonic macrophages situated in the thymus (Movies S9 and S10). |
|
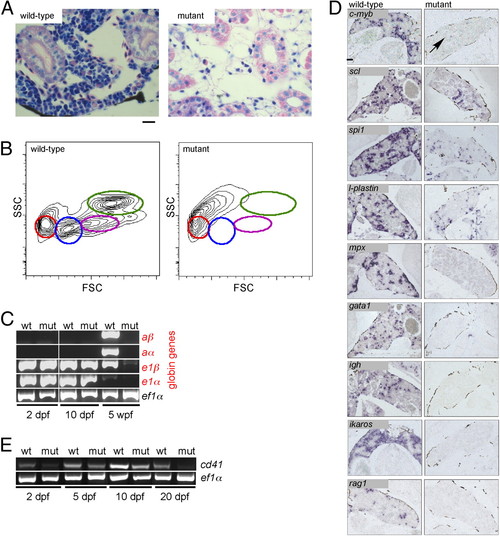
Failure of adult hematopoiesis in c-myb mutants. (A) Histological sections of the head kidney at 7 wk of age (Giemsa staining). Note the lack of hematopoietic cells in the mutant tissue. (Scale bar: 10 μm.) (B) Flow cytometric analysis of whole kidney marrow (6 wk of age) according to side scatter (SSC) and forward scatter (FSC) characteristics. Circles denote the positions of various cell types detectable in wild-type fish: red, erythrocytes; blue, lymphocytes and thrombocytes; magenta, precursors; green, myelomonocytes. The residual cells obtained from disintegrated mutant kidney tissue lack these characteristic features. (C) Lack of adult hemoglobin gene expression in c-myb mutants. RT-PCR was performed at the indicated time points for adult (prefix a) and embryonic (prefix e) globin genes; ef1α serves as a control for cDNA integrity and as a standard. (D) RNA in situ hybridization of head kidney sections was performed with the indicated probes. The signals seen with spi1 and l-plastin presumably originate from long-lived embryonic macrophages; a single c-myb positive cell is indicated (arrow). In mutant tissue, no signals are observed for mpx, gata1, ikaros, rag1, and igh. (Scale bar: 100 μm.) (E) Diminishing expression of cd41 in mutant embryos and larvae. RT-PCR was performed at the indicated time points; ef1α serves as a control for cDNA integrity and as a standard. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Phenotypic abnormalities in c-myb mutants. (A) Incomplete ossification in mutant fish (Lower; merged from three pictures) at 9 wk of age as revealed by alcian blue (cartilage) and alizarin red (red) staining. Note the stunted appearance and reduced bone structures. The photograph of the wild-type fish is a composite of two pictures. (Scale bar: 1 mm.) (B) Slightly reduced hemoglobin levels in mutant fish at 2 d postfertilization (dpf) as revealed by whole-mount o-dianisidine staining (orange color). Photographs are composites of three pictures. (C) Reduced hemoglobin levels in mutant fish at 9 dpf as revealed by whole-mount o-dianisidine staining. Photographs are composites of two pictures. (D) Lack of hemoglobin staining in the head kidney of mutants at 7 wk of age revealed by o-dianisidine staining on tissue sections. (Scale bar: 100 μm.) PHENOTYPE:
|
|
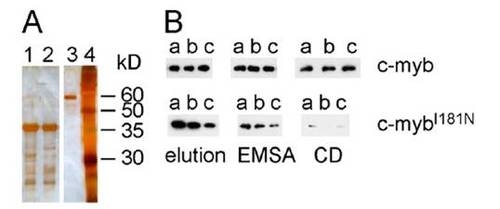
Solubility of the mutant c-myb DNA binding domain in vitro. (A) Purification of c-myb proteins from E. coli lysates after Ni-Sepharose affinity chromatography. Lane 1, wild-type protein; lane 2, mutant protein; lane 3, 60 kDa size standard (BSA); lane 4, molecular weight standards; sizes in kDa are indicated at the right. Silver stain. (B) Solubility of c-myb proteins was tested by Western blotting. Note that the CD buffer causes the protein to aggregate. |
|
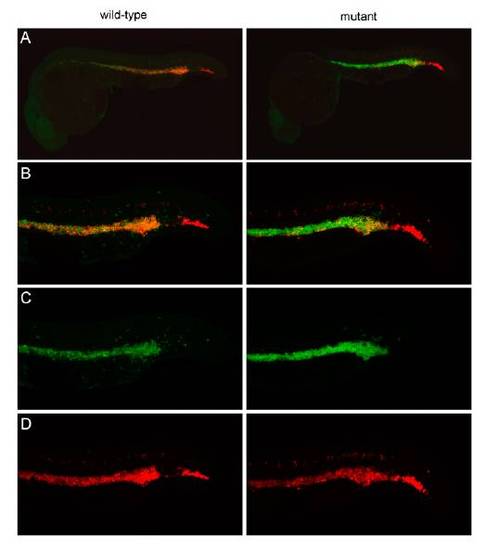
Characterization of hematopoietic progenitors in embryonic c-myb mutants at 24 h postfertilization (hpf). (A) Low power view of wild-type and mutant embryos simultaneously hybridized with probes specific for scl (red fluorescence) and c-myb (green fluorescence). The photograph of the wild-type embryo is merged from 45 optical slices at 5-μm thickness; the photograph of the mutant embryo is merged from 33 slices. (B) High-power view of A, focusing on the intermediate cell mass (ICM) and posterior blood island (PBI; this panel is also shown in Fig. 3A). The photograph of the wild-type embryo is merged from 22 optical slices at 5-μm thickness; the photograph of the mutant embryo is merged from 20 slices. (C) Same as in B but presenting only c-myb signals (green), indicating that c-myb levels are increased rather than the number of c-myb positive cells. (D) Same as in B but presenting only scl signals (red). |
|
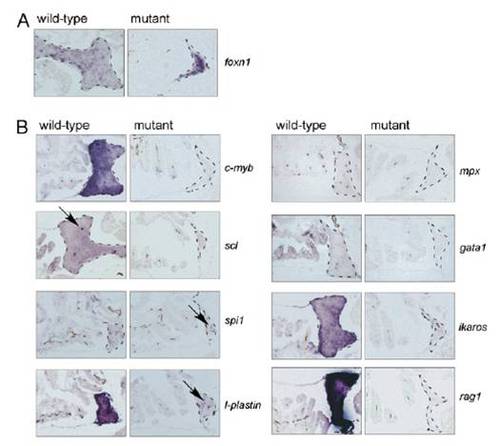
Lack of thymopoiesis in adult c-myb mutants. (A) The thymic epithelial anlage is present in c-myb mutants (age = 8 wk). Note that the epithelial cells are tightly clustered, because no hematopoietic cells are present in the tissue. No abnormality of the pharyngeal microenvironment was noted by alcian blue staining and foxn1-specific hybridization signals. The genotypes of fish are indicated. (B) Only long-lived macrophages are present in the mutant thymus (indicated by arrows in sections hybridized with spi1 and l-plastin); no other types of hematopoietic cells are present. Note that the wild-type thymus contains only very few cells positive for scl expression (arrow). RNA in situ hybridization of thymus sections with the indicated probes at 8 wk of age; similar results were obtained for 6 and 7 wk of age. The thymic rudiments are outlined by dashed lines. (Scale bar: 100 μm.) |