- Title
-
Dual function of perivascular fibroblasts in vascular stabilization in zebrafish
- Authors
- Rajan, A.M., Ma, R.C., Kocha, K.M., Zhang, D.J., Huang, P.
- Source
- Full text @ PLoS Genet.
|
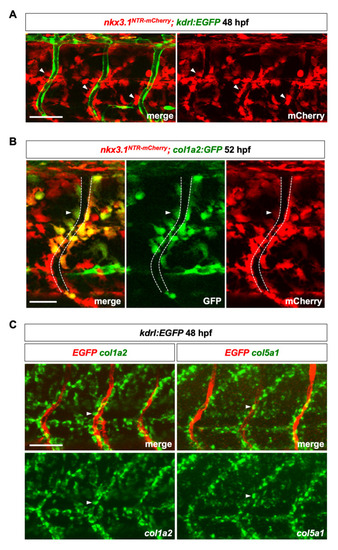
(A) Lateral view of a three somite region in EXPRESSION / LABELING:
|
|
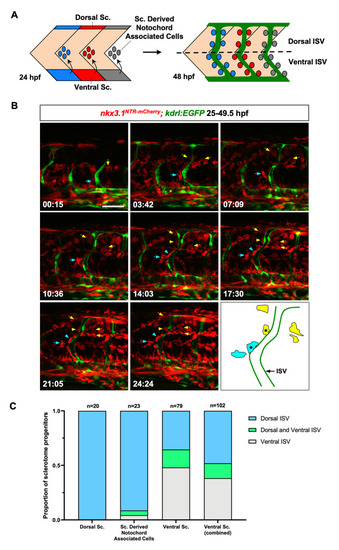
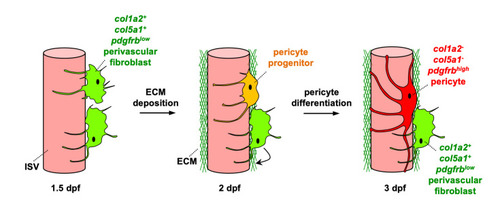
(A) Schematic representation of the bipartite organization of the zebrafish sclerotome and the generation of perivascular fibroblasts. At 24 hpf, the zebrafish sclerotome in each somite is divided into three compartments: the dorsal sclerotome, the ventral sclerotome, and sclerotome derived notochord associated cells. Note that notochord associated cells originate from the ventral sclerotome and are located about half-somite posterior to the corresponding somite. At 48 hpf, perivascular fibroblasts appear along the length of the intersegmental vessels (ISVs, green). The dotted line indicates the position of the horizontal myoseptum, which divides any given ISV into a dorsal half and a ventral half. Perivascular fibroblasts were quantified based on their final locations along each ISV: dorsal ISV (above the horizontal myoseptum) or ventral ISV (below the horizontal myoseptum). Sc: sclerotome. (B) Snapshots from time-lapse imaging of a |
|
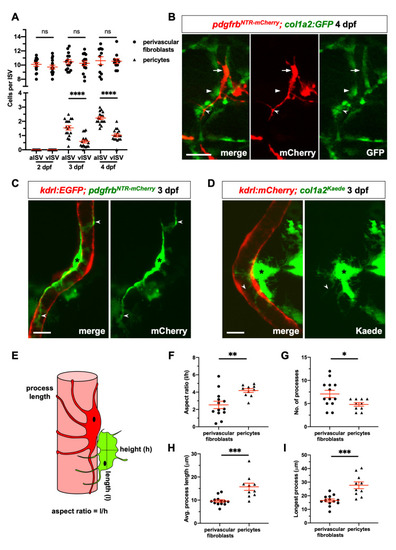
(A) Quantification of perivascular cell numbers along arterial ISVs (aISVs) and venous ISVs (vISVs) at 2, 3, and 4 dpf. The number of perivascular fibroblasts and pericytes were scored in EXPRESSION / LABELING:
|
|
(A) Snapshots from time-lapse imaging of PHENOTYPE:
|
|
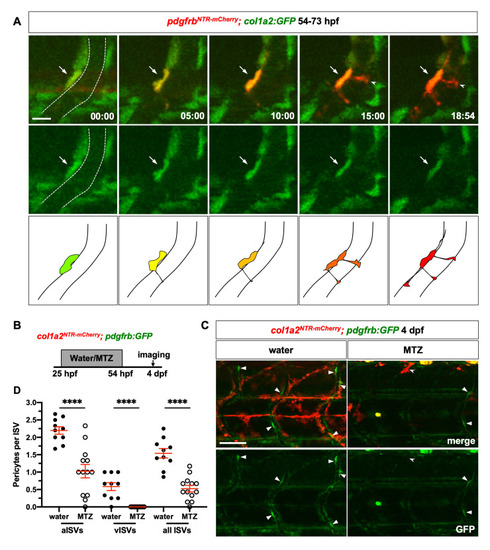
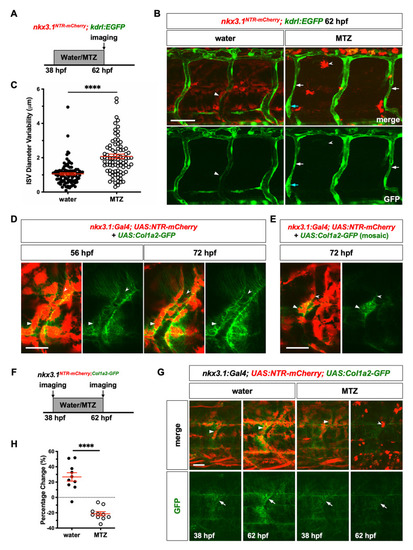
(A) Schematic of experimental protocol for early ablation of perivascular fibroblasts. PHENOTYPE:
|
|
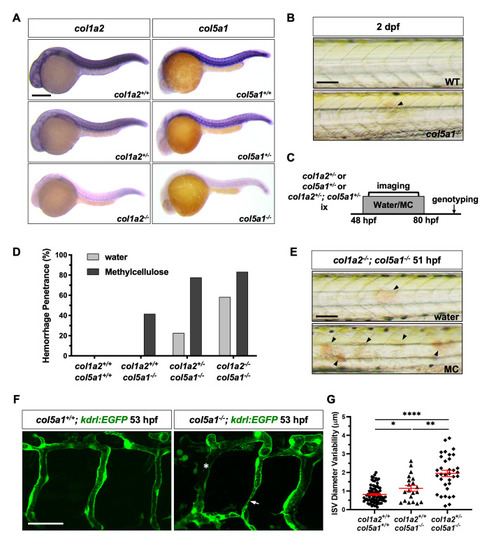
(A) Embryos from intercrosses of |
|
Perivascular fibroblasts, characterized by the expression of fibrillar collagens |