- Title
-
Regulation of the apical extension morphogenesis tunes the mechanosensory response of microvilliated neurons
- Authors
- Desban, L., Prendergast, A., Roussel, J., Rosello, M., Geny, D., Wyart, C., Bardet, P.L.
- Source
- Full text @ PLoS Biol.
|
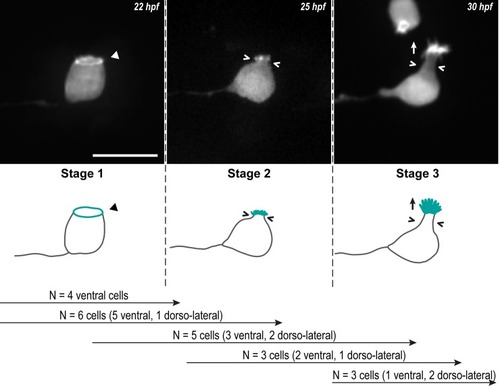
Z-projections from time-lapse acquisitions (top panels; lateral views of ventral CSF-cNs with rostral to the left) and schematics (bottom panels) showing the 3 stages CSF-cNs go through during the formation of their AE. The CSF-cN soma becomes round and short actin-based protrusions (Stage 2) arise from the ring of actin (Stage 1, arrowhead) concomitantly with a subapical constriction (Stages 2 and 3; chevrons). Gradually, protrusions lengthen to form the AE (Stage 3; arrow) characteristic of differentiated CSF-cNs. Data collected over 13 time-lapse sessions for 15 ventral and 6 dorsolateral cells. The number of cells imaged transitioning from one stage to the next is indicated in the bottom legend. Scale bar, 10 μm. AE, apical extension; CSF-cN, cerebrospinal fluid-contacting neuron. hpf, hours post fertilization. |
|
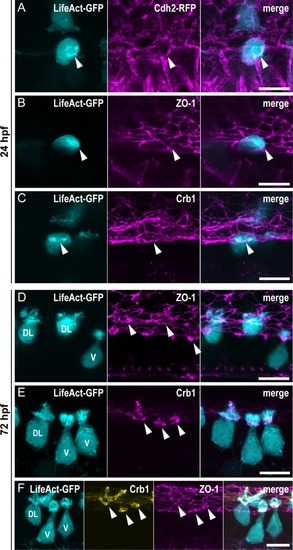
(A–C) Z-projections from lateral views of the spinal cord in 24-hpf embryos showing the colocalization of the ring of actin (LifeAct; arrowheads) with different markers of the AJCs: (A) Cdh2 for adherens junctions, (B) ZO-1 for tight junctions, and (C) Crb1 for the apical domain. (A) Double immunostaining for GFP and RFP in triple transgenic EXPRESSION / LABELING:
|
|
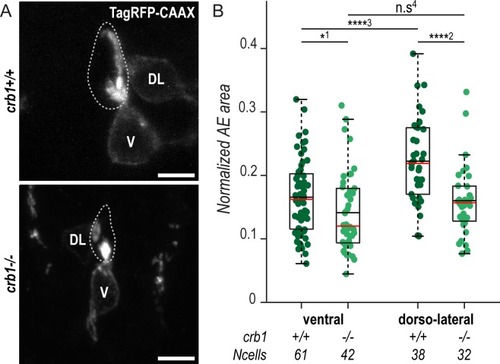
(A) Z-projections from transversal sections showing V and DL TagRFP-CAAX-expressing CSF-cNs in 72-hpf larvae illustrating the smaller AE in PHENOTYPE:
|
|
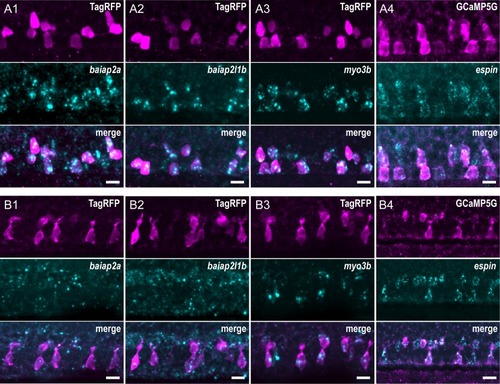
Expression of candidate factors known to be involved in the formation of actin-based protrusions— EXPRESSION / LABELING:
|
|
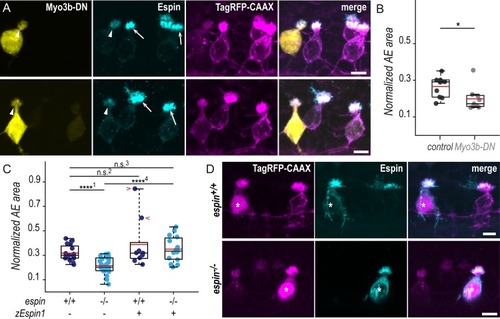
(A) Z-projections of whole-mounted spinal cords at 72 hpf showing mosaic expression of Myo3b-DN under the control of the PHENOTYPE:
|
|
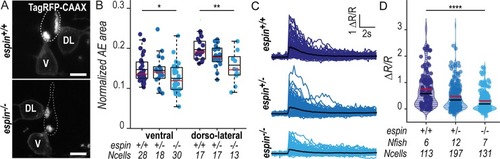
(A) Z-projections from transversal sections of spinal cords with V and DL TagRFP-CAAX-positive CSF-cNs at 72 hpf illustrating the smaller AEs in |