- Title
-
Miconazole protects blood vessels from matrix metalloproteinase 9-dependent rupture and hemorrhage
- Authors
- Yang, R., Zhang, Y., Huang, D., Luo, X., Zhang, L., Zhu, X., Zhang, X., Liu, Z., Han, J., Xiong, J.W.
- Source
- Full text @ Dis. Model. Mech.
|
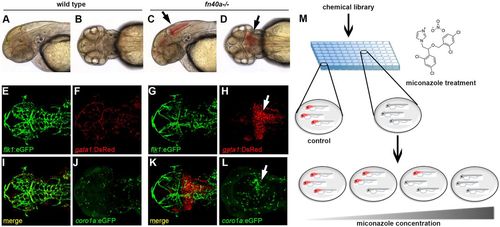
The fn40a mutant is a hemorrhagic stroke model suitable for chemical suppressor screens in zebrafish. (See also Figs S1,S2). (A-D) Live images of wild-type siblings (A,B) and homozygous fn40a mutants (C,D) at 2 dpf; note evident brain hemorrhages in the mutant (arrows). A,C: lateral view; B,D: dorsal view. (E-L) Live images of Tg(flk1:eGFP); Tg(gata1:DsRed) double transgenic embryos of wild-type siblings (E,F,I,J) and fn40a mutants (G,H,K,L) at 2 dpf. Note that gata1+ erythrocytes leak from Tg(flk1:eGFP)-labeled vessels and form hematomas in the mutants (G-H,K; arrow) while erythrocytes remain within vessels in wild-type siblings (E,F,I). Tg(coro1a:eGFP)-labeled macrophages and neutrophils accumulate at the hemorrhagic site in this mutant (L, arrow) whereas leukocytes are evenly distributed in the brain of a wild-type sibling embryo (J) at 2 dpf (n>8). (M) Overview of the chemical screen with mutant embryos arrayed in a 96-well plate and treated with DMSO or compound, illustrating that miconazole suppresses brain hemorrhages in a dose-dependent manner compared with DMSO treatment. Red color indicates hemorrhagic mutants. |
|
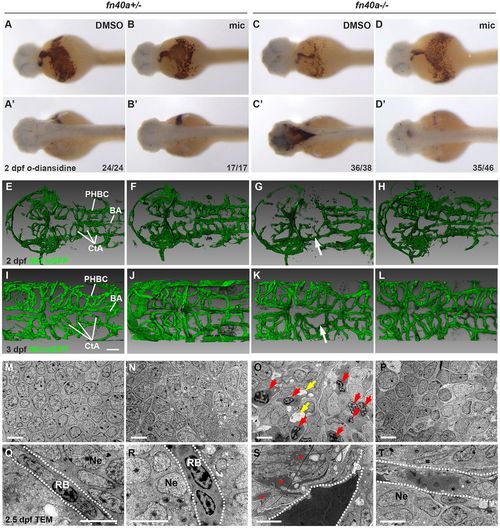
Miconazole suppresses brain hemorrhages in fn40a−/− mutants. (See also Figs S2-S4 and Table S1). (A-D′) O-dianisidine staining for erythrocytes showing hemorrhages in the dorsal part of the brain (C′) that were suppressed by miconazole (mic) treatment (D′) in homozygous fn40a mutants whereas no brain hemorrhages occur in heterozygous fn40a mutants in the absence or presence of miconazole (mic) (A-B′) at 2 dpf. A-D, ventral view; A′-D′, dorsal view. The numbers on the lower right show the number of hemorrhagic (C,C′) and non-hemorrhagic (A-B′,D,D′) embryos out of the total embryos scored. (E-L) CtA between BA and PHBC labeled in Tg(flk1:eGFP) zebrafish are disrupted (G,K, arrows) in homozygous mutants. This is rescued by mic treatment (H,L) in homozygous mutants compared with heterozygous mutants with either DMSO or mic treatment (E-F,I-J). Images are representative of more than 10 embryos per group (n>10). E-H, 2 dpf; I-L, 3 dpf. Scale bars: 50 µm. (M-T) Transmission electron microscopy shows the invasion of blood cells (red arrows) and disrupted neurons (O) and the leakage of high-density blood plasma (red asterisks) into the surrounding brain tissue (S) in fn40a homozygous mutants. This is rescued by mic treatment (P,T) compared with heterozygous mutants with or without mic treatment (M-N, Q-R) at 2.5 dpf. Images are representative of more than 8 embryos per group (n>8). In Q-T, dashed lines mark blood vessel border; red arrows indicate invaded blood cells; yellow arrows indicate vacuoles after cell death. BA, basilar artery; CtA, central artery; PHBC, primordial hindbrain channel; RB, red blood cell; Ne, neuron. Scale bars: 5 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
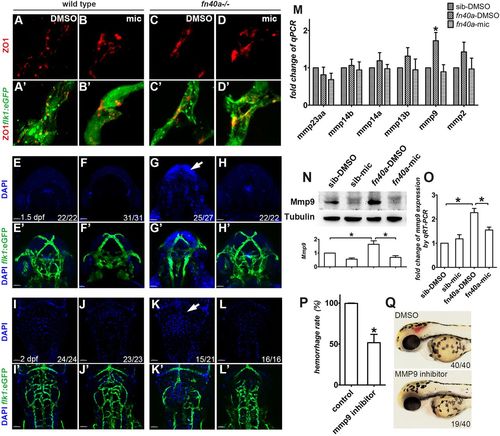
Miconazole suppresses brain hemorrhages by preventing Mmp9-mediated vessel disruption in fn40a−/− mutants. (See also Fig. S5). (A-D′) Fluorescence immunohistochemistry reveals comparable expression of the tight junction protein ZO1 in Tg(flk1:eGFP)-labeled brain vessels with or without miconazole (mic) treatment in wild-type siblings (A-B′) and fn40a mutants (C-D′) at 2 dpf. Images are representative of more than 15 embryos per group (n>15). (E-L′) Microinjection of DAPI into the sinus venous revealed that neurons are stained by leaked DAPI before (G,G′, arrow) and after (K,K′, arrow) evident hemorrhages, suggesting that vascular permeability increases in homozygous fn40a mutants. This was rescued by mic treatment (H,H′,L,L′), compared with much less leakage of DAPI in siblings with or without mic treatment (E-F′,I-J′). E-H′ are embryos at 1.5 dpf, I-L′ are embryos at 2 dpf. The numbers on the lower right show the number of phenotypical embryos out of the total embryos scored. Scale bars: 100 µm. (M) Quantitative real-time PCR identified that mmp9, but not other MMP genes, is upregulated in homozygous fn40a mutants and that was suppressed to the normal level by mic treatment at 2 dpf. *P<0.05 by two-way ANOVA with Bonferroni post-tests, compared with sib-DMSO group for each gene; means±s.e.m. (N,O) Western blot (N) and qRT-PCR (O) confirmed that both MMP9 protein and mmp9 mRNA increases in the mutants with DMSO control treatment and this is normalized by mic treatment, compared with wild-type siblings with or without mic treatment at 2 dpf. *P<0.05 by one-way ANOVA with Bonferroni's multiple comparison test, means±s.e.m. Total RNA was extracted from whole embryos and protein from whole embryos without yolk. (P,Q) MMP9 inhibitor I suppresses brain hemorrhage rate to 50% in homozygous mutants (P), in which brain hemorrhage was scored in live mutant embryos treated with DMSO or MMP9 inhibitor (Q). *P<0.05 by Student's t-test, compared with controls; means±s.e.m. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
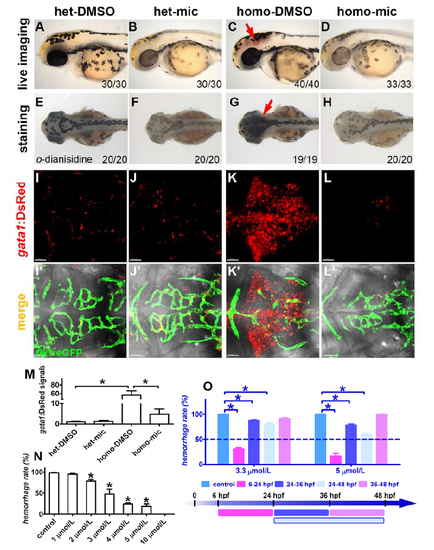
Miconazole suppresses brain hemorrhages in fn40a mutants. (A-D) Live image of embryos without PTU treatment. The homozygous fn40a mutant showed evident brain hemorrhage in the midbrain and hindbrain (C, arrow) compared with heterozygous mutant in the absence (A) or presence (B) of miconazole, and the hemorrhage was repressed in homozygous mutants by miconazole treatment (D). Embryos at 2 dpf were orientated in the lateral view. (E-H) O-dianisidine staining of embryos without PTU treatment revealed that erythrocytes accumulated in the brain region of homozygous fn40a mutant (G, arrow) compared with heterozygous mutant in the absence (E) or presence (F) of miconazole, and this hemorrhage phenotype in homozygous mutants was rescued by miconazole treatment (H). Embryos at 2 dpf were orientated in the dorsal view. The numbers on lower right show the phenotypical ones out of total scored embryos. (I-L, I'-L') Live images of Tg(flk1:eGFP; gata1:DsRed) double transgenic embryos of heterozygote siblings (I-J, I'-J') and homozygous fn40a mutants (K-L, K'-L') at 2 dpf in the hindbrain region. Note that gata1+ erythrocytes leaked out from Tg(flk1:eGFP)-labeled vessels and formed hematomas in the mutants (K, K') while erythrocytes remained within vessels in heterozygous siblings (I-J, I'-J') and mic-treated homozygous mutants (L, L'). (M) Intensity of the gata1:DsRed signals in the hindbrain region shown in panels A-D, noting a burst of gata1:DsRed signals in homozygous fn40a mutants compared with heterozygous mutants, which was reversed in homozygous mutants after mic treatment. *p <0.05 by one-way ANOVA with Bonferroni's Multiple Comparison Test, n>10. (N) Miconazole suppresses brain hemorrhages in fn40a mutants in a dose-dependent manner. Note that miconazole at 10 µmol/L completely suppressed brain hemorrhage in fn40a mutants. *p <0.05 by one-way ANOVA with Dunnett's Multiple Comparison Test, compared with control, mean±SEM., n >50 for each independent repeat. (O) Application of miconazole from 6 to 24 hpf had more effective suppression on brain hemorrhage in fn40a mutants. At the concentration of 3.3 μmol/L, miconazole effectively suppressed brain hemorrhage (more than 50%) when its treatment from 6 to 24 hpf, but had little effect when its treatment either from 24 to 36 hpf, or 24 to 48 hpf, and had no effect when having treatment from 36 to 48 hpf. Similarly, at the concentration of 5 μmol/L, miconazole inhibited brain hemorrhage of fn40a mutants (better than that at 3.3 μmol/L) when having treatment from 6 to 24 hpf, and had less inhibition when having treatment either from 24 to 36 hpf or from 24 to 48 hpf, but had no effect when having treatment from 36 to 48 hpf. *p <0.05 by two-way ANOVA with Bonferroni's post test compared with control, mean±SEM, n >100. |
|
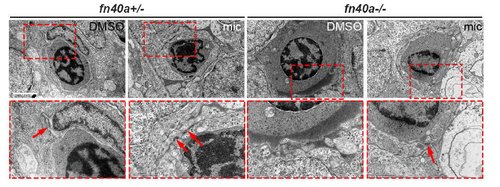
Ultrastructures of microvessels in the zebrafish brain shown by transmission electron microscopy at 2.5 dpf (refer to Fig. 2). (A-D) Endothelial cells wraps well around red blood cells in fn40a heterozygotes with or without miconazole treatment (A-B). Endothelial cells outside red blood cells were disrupted in fn40a homozygote (C), which was rescued by miconazole treatment (D). RB, red blood cell; E, endothelial cell; Ne, neuron; mic, miconazole. Arrows point to the tight junctions between endothelial cells. The lower panels are magnification of the red-square area in the upper ones. Number of embryos per group was more than 8. Scale bar, 2 μm. |
|
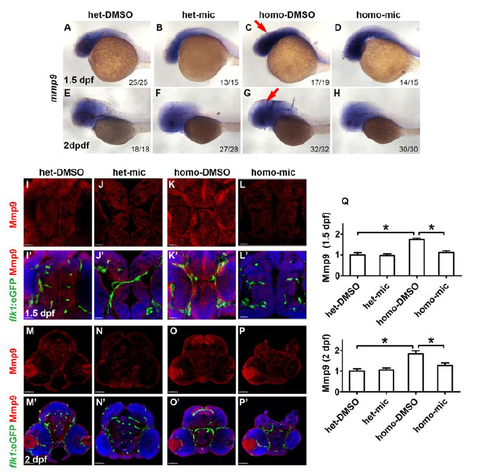
Miconazole inhibits Mmp9 elevation in the brain of fn40a mutants. (A-H) RNA in situ hybridization revealed an increase of mmp9 expression in the brain region of fn40a mutants at 1.5 dpf (C) and 2 dpf (G) compared with heterozygote controls (A, E), and decreased mmp9 expression in fn40a mutants after mic treatment (D, H). A-D, 1.5 dpf; E-H, 2 dpf. A-H, lateral view. Red arrows point to the brain. (I-P, I'-P') Fluorescence immunohistochemistry showed a higher level of Mmp9 (red) in the brain of homozygous fn40a mutants (K, K'; O, O') compared with heterozygous controls in the absence (I, I'; M, M') or presence (J, J'; N, N') of miconazole; and miconazole decreased the Mmp9 level in homozygous mutants (L, L'; P, P'). Tg(flk1:eGFP) (green) was used for labeling brain vessels and DAPI (blue) for staining the nuclei. I-L, I'-L', 1.5 dpf, scale bar is 20 μm; M-P, M'- P', 2 dpf, scale bar is 40 μm. (Q) Intensity of anti-Mmp9 staining signals in the brain regions shown in panels I-P, confirming that the elevated expression of Mmp9 in fn40a mutants was suppressed by miconazole treatments. *p <0.05 by one-way ANOVA with Bonferroni's Multiple Comparison Test. n >10 for each zebrafish independent repeat. |
|
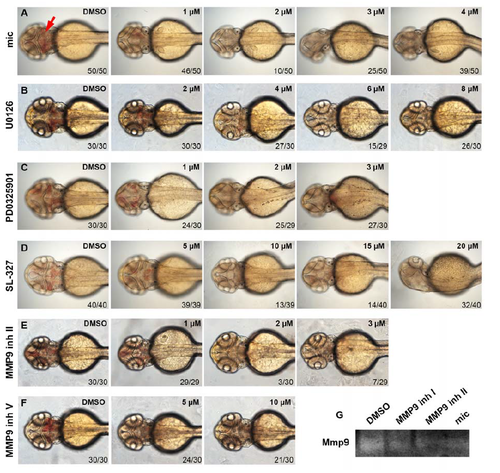
Either MMP9 or pErk inhibitors decrease brain hemorrhage in fn40a mutants. Live imaging is shown for fn40a embryos treated by DMSO or small-molecule inhibitors. The hemorrhage rate of fn40a mutants was suppressed by miconazole (1 to 4 µmol/L (μM) mic, A), MEK inhibitor (2 to 8 µmol/L U0126, B; 1 to 3 µmol/L PD0325901, C; and 5 to 20 µmol/L SL-327, D), or MMP9 inhibitor II (1 to 3 µmol/L, E) and MMP9 inhibitor V (5 to 10 µmol/L, F) compared control embryos treated with DMSO. Embryos were orientated anterior on left with the dorsal view. Red arrow indicates the hemorrhage region. The concentrations of each compound were shown on upper right. The numbers on lower right show the phenotypical ones out of total scored embryos. (G) MMP9 zymography assay revealed that either MMP9 inhibitor I (3 µmol/L), MMP9 inhibitor II (3 µmol/L), or miconazole (3 µmol/L) decreased Mmp9 activities of homozygous fn40a mutants compared with DMSOtreated mutants. This represents one of two independent experiments. |
|
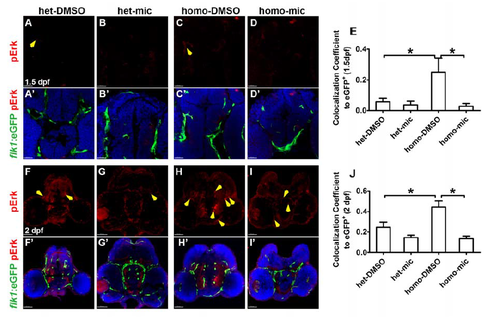
Miconazole inhibits pErk elevation in the brain vessels of fn40a mutants. Fluorescence immunohistochemistry showed that the phosphorylated Erk1/2 (pErk, red) in Tg(flk1:eGFP) endothelial cells (green) increased in homozygous fn40a mutants (C, C'; H, H') compared with heterozygous siblings in the absence (A, A'; F, F') or presence (B, B'; G, G') of miconazole; and pErk decreased in homozygous mutants after mic treatments (D, D'; I, I'). Tg(flk1:eGFP) (green) was used to label vascular endothelia cells. The colocalization coefficient of pErk and flk1:eGFP to eGFP signals was shown in panel E (1.5 dpf) and panel J (2 dpf), noting that the pErk was up-regulated in mutant endothelium and the elevated pErk was rescued by miconazole treatments. A-D, A'-D', 1.5 dpf, scale bar is 20 μm; F-I, F'-I', 2 dpf, scale bar is 40 μm. DAPI (blue) was used to label nuclei. Yellow arrows indicated the doublepositive signals (pErk/flk1:eGFP). *p <0.05 by one-way ANOVA with Bonferroni's Multiple Comparison Test, n >10 for each zebrafish independent repeat. |