- Title
-
Differential Lectin Binding Patterns Identify Distinct Heart Regions in Giant Danio (Devario aequipinnatus) and Zebrafish (Danio rerio) Hearts
- Authors
- Manalo, T., May, A., Quinn, J., Lafontant, D.S., Shifatu, O., He, W., Gonzalez-Rosa, J.M., Burns, G.C., Burns, C.E., Burns, A.R., Lafontant, P.J.
- Source
- Full text @ J. Histochem. Cytochem.
|
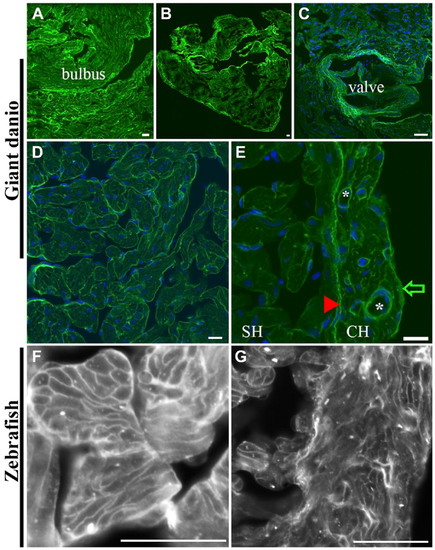
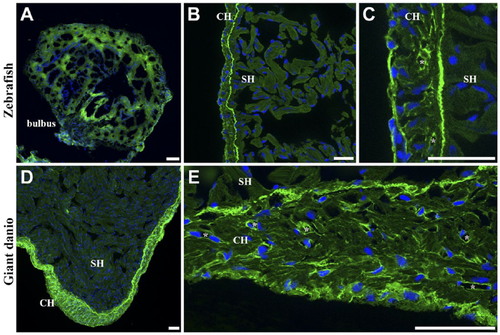
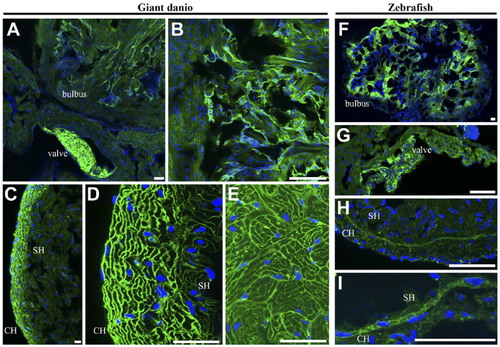
Differential Con A lectin binding in the giant danio and zebrafish hearts. Con A-fluorescein lectin binding (green) is observed throughout the giant danio heart with the strongest staining seen on the bulbus (A), the CH (B), and the valves (C; nuclei, blue). At higher magnification, Con A binds to the trabecular bundles in the spongy myocardium and to cardiomyocyte membranes (D). Con A binding is comparatively stronger in the CH compared with that in the SH (E). Within the compact myocardium, Con A binding is particularly strong in the epicardium (arrow) and the coronary vasculature (*). The junctional region (arrowhead) is equally stained, forming a boundary line between the CH and SH, with small regions of discontinuity at regular intervals. A similar pattern is seen in the zebrafish heart with Con A staining cardiomyocyte borders of the SH (F) and CH (G). Abbreviations: Con A, concanavalin A; SH, spongy heart; CH, compact heart. Scale bars: A–G = 10 µm. |
|
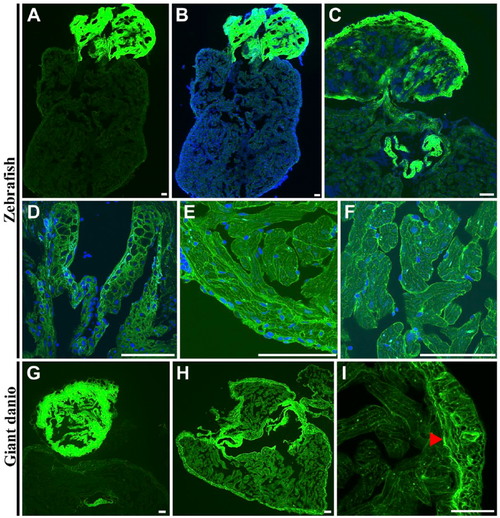
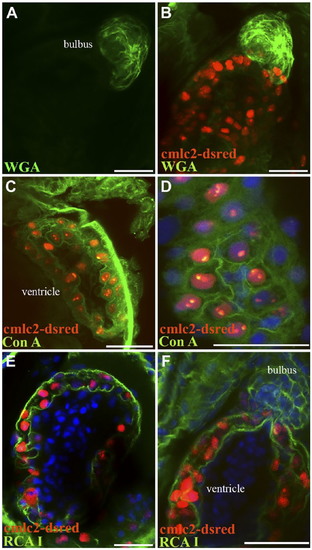
Differential wheat germ agglutinin (WGA) lectin binding in the zebrafish and giant danio hearts. In the zebrafish, the strongest WGA lectin binding was observed in the bulbus mesenchyme (A, B) with the ventricle appearing minimally stained by comparison, and in the valves (C, D). At higher magnification, WGA could be seen staining the compact heart, the trabecular bundles of the spongy heart (E), and the cardiomyocyte borders (F). In the giant danio, the staining pattern was similar with the strongest staining level in the bulbus mesenchyme (G) with the ventricle appearing minimally stained. When imaged without the bulbus, WGA staining is seen throughout the ventricle but with higher levels in the compact heart and the valves (H). At higher magnification, WGA was observed to stain cardiac myocyte borders as well as the junctional region (arrowhead) of the giant danio heart (I). Scale bars: A–I = 50 µm. |
|
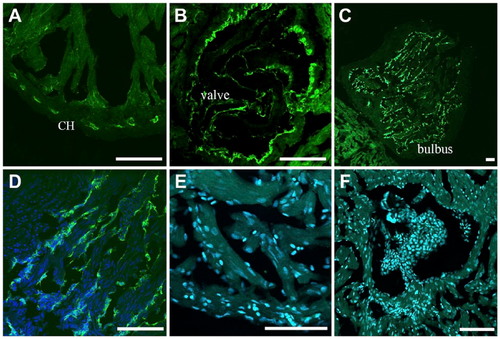
Differential BS lectin binding in the giant danio and zebrafish hearts. BS lectin strongly stained the endothelium of the coronary vasculature of the CH, with minimal binding seen in the ventricular endocardium (A). Strong BS lectin binding was seen in the endothelium of the valves (B). BS lectin strongly stained the bulbus (C). Higher magnification show that the staining was localized in the bulbus endothelium folds (D). No BS lectin binding was observed in the zebrafish ventricle (E), including the valves (F). Abbreviations: BS, Bandeiraea simplicifolia; CH, compact heart. Scale bars: A–F = 50 µm. |
|
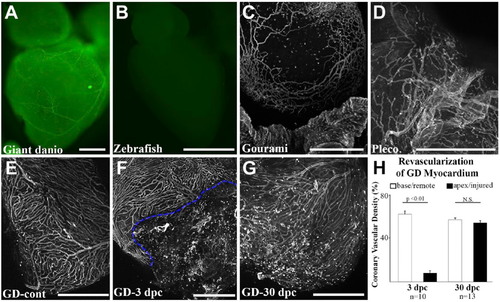
Whole-mount staining and imaging of fish coronary vasculature. Widefield imaging of Bandeiraea simplicifolia (BS) lectin-fluorescein stained adult giant danio heart where large and small coronary vessels can be visualized (A). Widefield imaging of BS lectin-fluorescein stained zebrafish heart showing absence of BS lectin staining (B). Maximum projection of optical sections showing a vascular network on the gourami bulbus (C) and in the pleco ventricle (D). Maximum projection of optical sections showing a vascular network of uninjured giant danio heart (E) and during regeneration at days 3 (F) and 30 (G) following apical ventricular cautery injury. Dashed line highlights the border zone between injured and non-injured areas of the ventricle. Quantitation of coronary vascularization (H) during regeneration. Scale bars: A–G = 500 µm. GD = giant danio. |
|
Differential RCA I lectin binding in giant danio and zebrafish hearts. Low level of RCA I lectin staining was seen in the zebrafish bulbus mesenchyme (A). Strong RCA I lectin binding is seen in the zebrafish CH, and particularly in the junctional region (B). At higher magnification, RCA I staining can be observed in the epicardium, cardiomyocyte borders, and in coronary vessel profiles, albeit at a lower level than the junctional region (C; *, vessel). A similar binding pattern was seen in the giant danio with strong binding in the CH (D) and little to no reactivity in the spongy myocardium. At higher magnification, RCA I is observed to bind strongly in the epicardium, the junctional region, cardiomyocyte borders, and coronary vessel profiles (E; *, vessel). Abbreviations: RCA I, Ricinus communis agglutinin I; SH, spongy heart; CH, compact heart. Scale bars: A–E = 20 µm. |
|
Differential tomato lectin binding in giant danio and zebrafish hearts. Tomato lectin staining was present in the bulbus endothelium and mesenchyme, and strong in the valves (A, B; higher magnification of bulbus) of the giant danio. Binding was also strong in the CH, staining the cardiomyocyte borders (C, D; higher magnification). A similar binding pattern was seen in the SH but weaker (E). In the zebrafish, tomato lectin staining was present in the bulbus (F) and the valve (G). Little to no reactivity was seen in the ventricle except for low but discernable binding in the junctional region (H) and to the borders of the transitional cardiac myocytes (I) bridging the compact and spongy myocardia. Abbreviations: SH, spongy heart; CH, compact heart. Scale bars: A–I = 20 µm. |
|
Differential lectin binding in developing zebrafish heart. WGA lectin staining of zebrafish heart 72 hr postfertilization shows strong staining of the bulbus mesenchyme (A, B), and little to no staining of the ventricle. Con A stains the endocardium and epicardium of the developing heart (C) as well as cardiac myocyte borders (D). RCA I stains strongly the epicardium and myocardium (E) as well as the developing bulbus (F). Abbreviations: WGA, wheat germ agglutinin; Con A, concanavalin A; RCA I, Ricinus communis agglutinin I. Scale bars: A–F = 10 µm. |