- Title
-
A Complex of BBS1 and NPHP7 Is Required for Cilia Motility in Zebrafish
- Authors
- Kim, Y.H., Epting, D., Slanchev, K., Engel, C., Walz, G., and Kramer-Zucker, A.
- Source
- Full text @ PLoS One

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
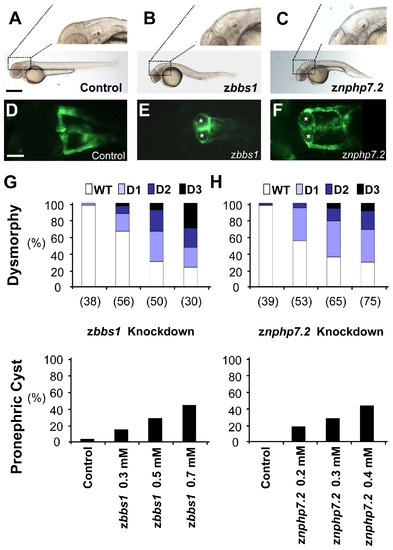
Depletion of zBbs1 and zNphp7.2 causes dose-dependent hydrocephalus and pronephric cysts. Zebrafish embryos injected with MOs at the 1-cell stage were evaluated for their phenotype at 55 hpf. (A) Control zebrafish embryo with magnified normal brain area. Scale bar = 500 μm. (B and C) zbbs1 AUG MO-injected embryo and znphp7.2 SP1 MO-injected embryo with hydrocephalus. (D) The Tg(WT1b:EGFP) transgenic line shows normal pronephric glomerulus and tubules (Scale bar = 100 μm), (E and F) zbbs1 AUG MO-injected embryo and znphp7.2 SP1 MO-injected embryo show pronephric cysts (asterisks). At 55 hpf, larval dysmorphy was categorized according to the visual scale (Fig. S3). The degree of dysmorphy was increased with the amount of injected MOs (G, zbbs1 AUG MO; H, znphp7.2 SP1 MO). (G and H) Cystic pronephric phenotypes were dose-dependent in zebrafish embryos injected with zbbs1 and znphp7.2 MO respectively. The graphs show percentages of the number (n) of embryos that were examined. |
|
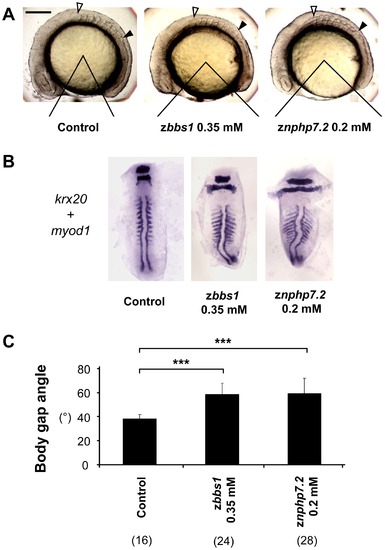
zBbs1 and zNphp7.2 morphant embryos showed defects in convergent extension movements. (A) Representative side views of control embryos and morphants demonstrate the analysis of the body gap angle which is inversely related to length of the body. Embryos were randomly picked at 8–10 somite stage. Open arrowhead marks first somite, filled arrowhead ninth somite respectively. Scale bar = 200 µm. (B) The expression of krx20/myod1 in morphant embryos showed shortened body axis with significantly broader somites compared to control. (C) The morphants of zbbs1 and znphp7.2 showed wider body gap angle compared to control embryos. The numbers in the brackets (n) are the numbers of total embryos which were examined. PHENOTYPE:
|
|
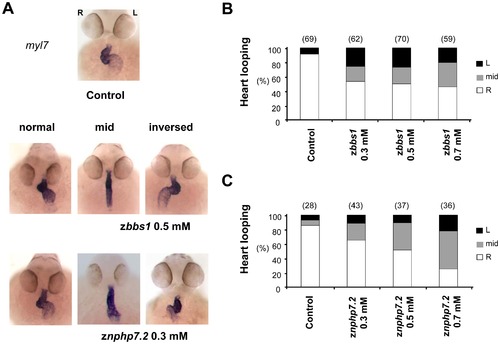
Depletion of zBbs1 and zNphp7.2 caused defects in heart looping. To examine organ laterality defects, zebrafish embryos were examined for changes in heart looping. (A) In situ hybridisation (myosin light chain 7; myl7) of both zbbs1 and znphp7.2 morphant embryos at 55 hpf showed defective heart looping. (heart looping to the right (R = normal), without heart looping (mid) and looping to the left (L = inversed)) (B) Whereas the hearts of control embryos showed more than 90% rightward looping, approximately 50% of zBbs1-depleted embryos and (C) 35–75% of zNphp7.2-depleted embryos had situs inversus or mid position of the heart. The numbers in the brackets (n) are the numbers of total embryos which were examined. |
|
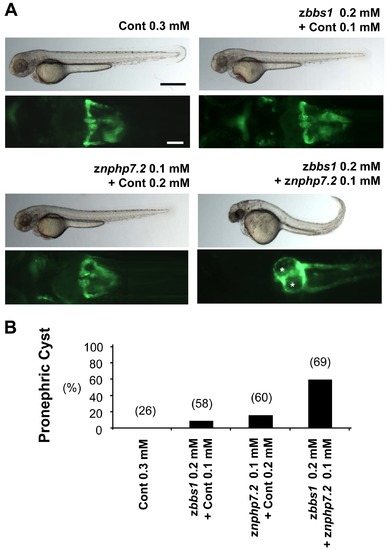
Genetic interaction between zbbs1 and znphp7.2 in vivo. Injected zebrafish embryos were assessed for the incidence of pronephric cysts. (A) Cont MO injected embryos with normal pronephros. While the majority of zbbs1 or znphp7.2 morphants (suboptimal dose) showed pronephros of normal morphology, those of combined knockdown exhibited pronephros with cysts. Cysts are marked by asterisks. (Black scale bar = 500 μm, White scale bar = 100 μm) (B) Pronephric cysts were detectable after individual injections of znphp7.2 MO at 0.1 mM and zbbs1 MO at 0.2 mM with the level of 17% and 10% respectively whereas the combined knockdown caused pronephric cysts in 59% of microinjected embryos. The final MO concentration for injection was 0.3 mM in all conditions to keep total MO dose constant. Therefore suboptimal doses of zbbs1 and znphp7.2 MO were combined with Cont MO to obtain this final concentration of 0.3 mM. The numbers in the brackets (n) are the numbers of total embryos which were examined. |
|
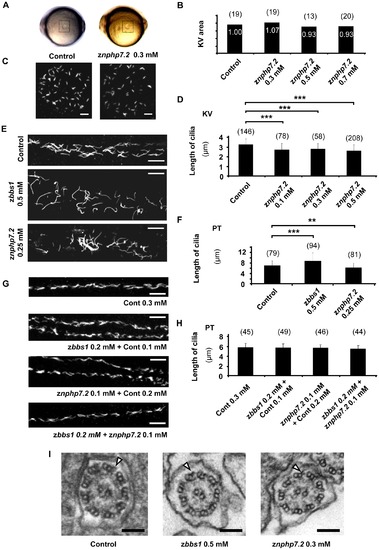
Knockdown of znphp7.2 showed normal development of cilia. (A) Images of live zebrafish embryos at the 8–10 somite stage. Kupffer′s vesicle is located in the dashed box. (B) Measurement of relative KV area with setting control as ‘1.00’. The knockdown of znphp7.2 did not significantly affect on KV development and area. (C) Images of cilia in the KV of 8–10 somite stage were stained for acetylated tubulin. Scale bar = 10 μm. (D) zNphp7.2-deficient embryos showed shortened length of cilia in KV compared to control embryos. (E) Staining of acetylated tubulin in the anterior pronephric tubule of morphants at 48 hpf displayed that the overall distribution of cilia remained unchanged compared to control (Scale bar = 10 μm) even though (F) the knockdown either of zbbs1 showed longer and the knockdown of znphp7.2 showed shorter cilia. (PT, Pronephric Tubule) (G and H) The morphology and length of cilia in posterior pronephric tubule appeared unchanged in combined knockdown of zbbs1 and znphp7.2 compared to single knockdown of zbbs1 or znphp7.2 or compared to Cont MO injected embryos. (PT, Pronephric Tubule) (I) The morphants of zbbs1 and znphp7.2 displayed normal ultrastructure of motile cilia in pronephric tubule without recognizable deficiency in dynein arms (outer dynein arm marked by arrowhead). The numbers (n) in the graphs are the number of total cilia which were examined. 4–6 individual embryos per group were examined. PHENOTYPE:
|
|
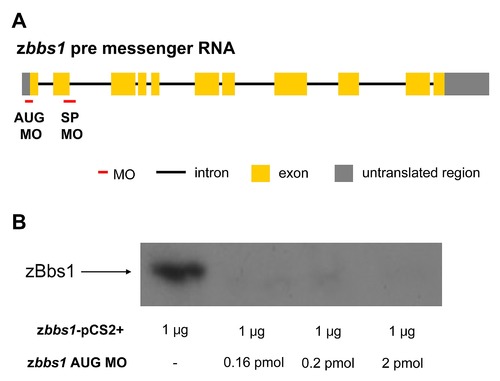
Antisense morpholino oligonucleotides against zbbs1. (A) MO (red line) targeting zbbs1 translational start codon (AUG MO) and MO targeting exon 2 splice donor site (SP MO) of zbbs1. (B) 1 µg of plasmids DNA containing wild-type full-length zbbs1 in pCS2+ vector was mixed with or without MOs in vitro translation reactions. zbbs1 AUG MO efficiently interfered with zBbs1 protein expression. |
|
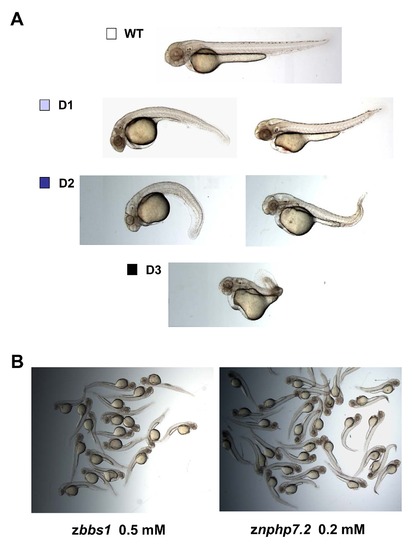
Grading of dysmorphy. (A) Embryos at 48–55 hpf were assessed and scored as wild-type-like or with a degree of dysmorphy ranging from mild (D1) to severe (D3). (B) zbbs1 or znphp7.2 morphant embryos were always consist of a mixed population of individuals with dorsally or ventrally curved body axis. PHENOTYPE:
|
|
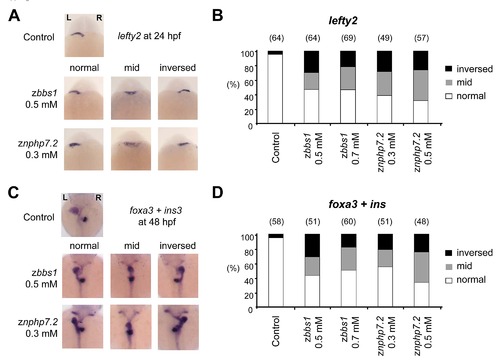
zBbs1- or zNphp7.2-deficient embryos showed defects in organ laterality. (A, B) In situ hybridisation of both zbbs1 and znphp7.2 morphants at 24 hpf with lefty2 probe showed defective left-right asymmetry patterning. (C and D) Defective laterality of liver (foxa3) and pancreas (ins) was observed in zBbs1- and zNphp7.2-depleted embryos at 48 hpf. |
|
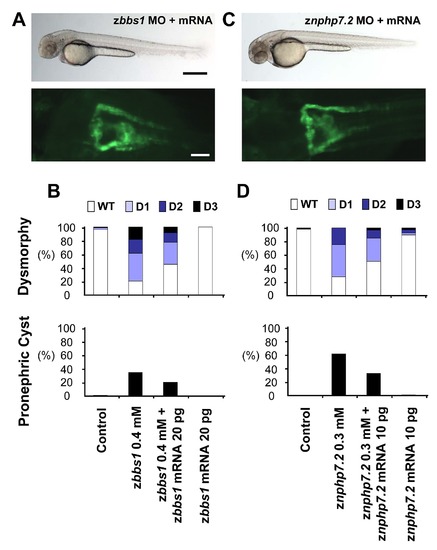
Co-Injection of zbbs1 or znphp7.2 mRNA partially rescued the corresponding morphant phenotype. Co-injection of 20 pg of zbbs1 mRNA (A and B) or 10 pg of znphp7.2 mRNA (C and D) together with the corresponding MO partially rescued the dysmorphic changes caused by the MO-mediated knockdown, decreasing the dorsal body curvature and rescued pronephric cyst formation. (A: scale bar = 500 µm, B: scale bar = 100 µm) (D, X2 = 5.27, P = 0.022; H, X2 = 14.5, P<0.001). |
|
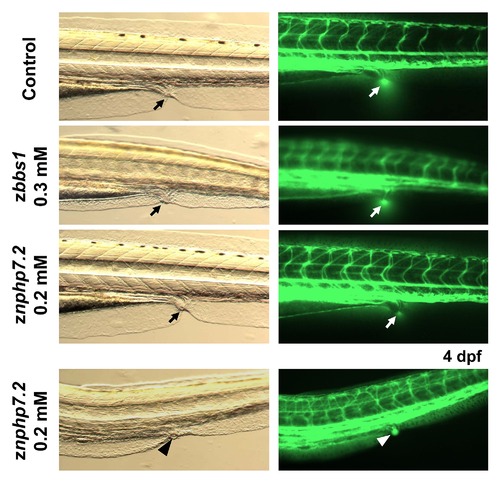
Dextran injection revealed fluid excretion via the cloaca in zbbs1 and znphp7.2 morphants. Zebrafish control and morphant embryos with pronephric cysts at 96 hpf were injected with 5% FITC-conjugated dextran solution (70 kD) into the circulation. Fluorescent dye excretion with the urine at the cloaca (black and white arrows) was observed in control embryos (22/22), zbbs1 (16/16) and znphp7.2 (21/23) morphants. The lower panel represents a znphp7.2 morphant embryo with missing fluorescent dye excretion due to persistent closure of the cloaca (arrowheads). Images on the left column represent transmitted light images and images on the right column represent fluorescent images of the same embryo for each setting. PHENOTYPE:
|
|
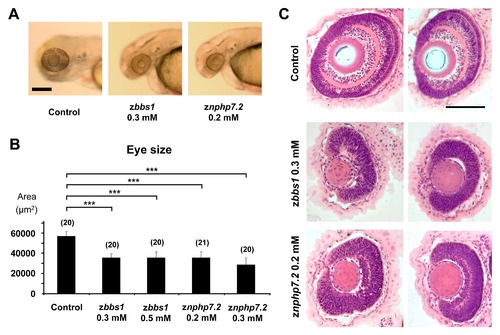
zbbs1 and znphp7.2 morphants display reduced eye size and defective retinal layer formation. The eye size as area (μm2) was measured for control embryos, zbbs1 and znphp7.2 morphant embryos at 80 hpf. (A) Representative brightfield images showing reduced eye size for zbbs1 and znphp7.2 morphants in comparison to the control (Scale bar = 200 μm). (B) Statistical quantification of the measurements proved that the reduction in eye size for zbbs1 and znphp7.2 morphants was significant in comparison to the control. (C) Histological cross-sections of 96 hpf zbbs1 and znphp7.2 morphants revealed defective layer formation in comparison to the control. Two representative images are shown for each setting. (Scale bar = 100 μm). PHENOTYPE:
|
|
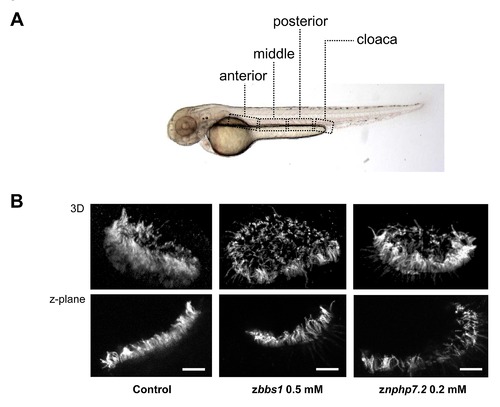
The morphants of zbbs1 and znphp7.2 exhibited impaired motility of cilia in the pronephric tubule. (A) The areas where the movies were recorded are shown by the dashed box. (B) Acetylated tubulin staining demonstrated normal development of cilia in the nasal pit (Scale bar = 10 µm). 3-dimensional (3D) images (upper panel) and z-plane images (lower panel) show that the cilia formation in the morphants of zbbs1 and znphp7.2 is normal compared to control embryo. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|