- Title
-
Heterogeneity across the dorso-ventral axis in zebrafish EVL is regulated by a novel module consisting of sox, snail1a and max genes
- Authors
- Chen, Y.Y., Harris, M.P., Levesque, M.P., Nüsslein-Volhard, C., and Sonawane, M.
- Source
- Full text @ Mech. Dev.
|
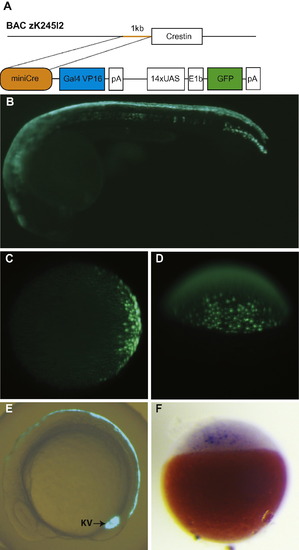
The miniCrestin promoter drives Gal4-mediated GFP expression in the dorsal EVL and Kupffer’s vesicle. (A) A 1 kb promoter in front of the crestin open reading frame was isolated by PCR from zebrafish BAC zK245I2, ligated to a Gal4–UAS–GFP transgene construct and injected to zebrafish embryos at the 1-cell stage to create the transgenic line. (B) At 24hpf, the transgene is mostly expressed in the dorsal periderm. (C and D) GFP expression in the transgenic embryo is first detectable at 30% epiboly stage in the future dorsal domain. (C) Animal pole view. (D) Dorsal view. (E) A GFP labelled population contributes to the dorsal periderm and Kupffer’s vesicle at the 10-somite stage. (F) In situ hybridization analysis shows that Gal4 is expressed prior to GFP expression at the sphere stage. EXPRESSION / LABELING:
|
|
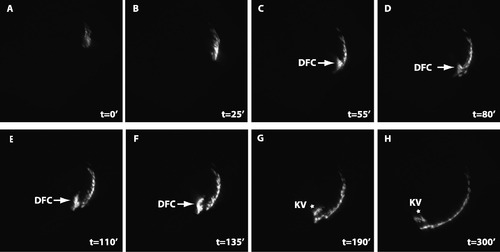
Dorsal forerunner cells ingress from dorsal EVL to form Kupffer’s vesicles. Time lapse imaging shows that marginal dEVL cells ingress to form dorsal forerunner cells (DFC, arrows), which subsequently form Kupffer’s vesicle (KV, stars). The total duration of the movie is 300 min. The imaging started at the 50% epibody stage (A, t = 02). The interval between each frame is 5 min. Dorsal is to the right. EXPRESSION / LABELING:
|
|
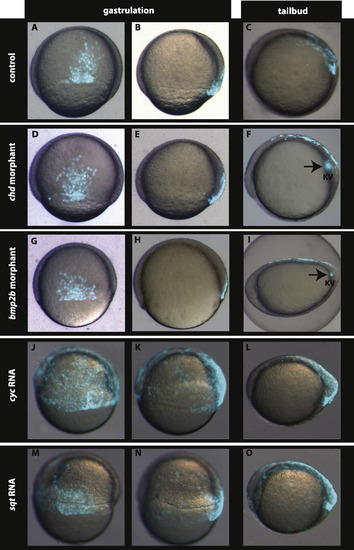
Nodal signalling is involved but Chordin–BMP signalling is dispensable in setting up the dorsal EVL (dEVL) GFP domain. Compared to the control (A and B), the GFP labelled dEVL domain is neither altered in chd morphants (D and E) nor in bmp2b morphants (G and H). Interestingly, over-expression of nodal related ligands cyc (J and K) and sqt (M and N) leads to expansion of the dEVL as well as the dorsal forerunner cells. At tail-bud stage the GFP expression is restricted to the dorsal periderm and the Kupffer’s vesicle in control embryos (C). This GFP expression is not altered in chd (F) and bmp2b morphants (I) whereas expansion of the GFP domain in the periderm and in Kupffer’s vesicle are observed in embryos over-expressing cyc (L) and sqt (O). A, D, G, J, and M dorsal view at 60–70% epiboly. B, E, H, K, and N lateral view at 60–70% epiboly. Dorsal is to the right. C, F, I, L, and O lateral view, anterior is to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
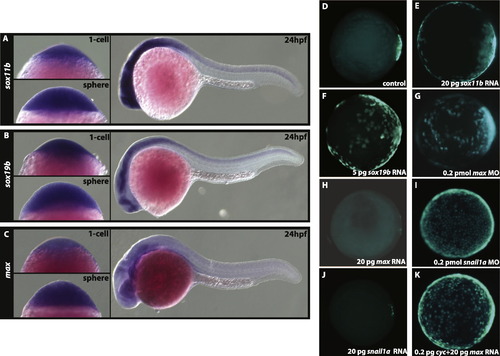
Expression analyses of sox11b, sox19b, and max. In situ hybridization of sox11b (A), sox19b (B), and max (C) at 1-cell, sphere, and 24hpf stages. All three genes are maternally contributed, ubiquitously expressed at sphere stage, and expressed in the CNS at 24 hpf (A–C). (D–K) Animal pole view of GFP expression in Tg (miniCrestin:Gal4, UAS:GFP) embryos at shield stage. In control embryos at the shield stage (D) GFP expression is restricted to the dorsal domain. This domain is expanded in sox11b (E), sox19b (F) over-expressing and max deficient (G) embryos. The over-expression of max (H) represses transgene expression in the dEVL. Similar to max, snail1a deficient embryos exhibit expansion (I) whereas over-expressing snail1a embryos (J) show reduction in the dEVL domain. The dEVL remains expanded in cyc and max mRNA co-injected embryos (K) indicating that Nodal signalling is sufficient to regulate dEVL formation. |
|
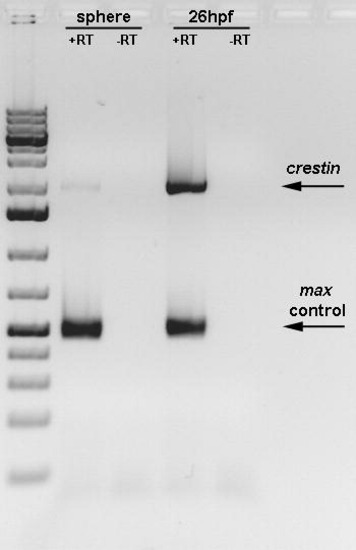
RT-PCR of crestin gene expression at sphere stage and 26 hpf. The crestin gene is expressed at low levels at the sphere stage. +RT- in presence of reverse transcriptase; RT- in absence of reverse transcriptase. |
|
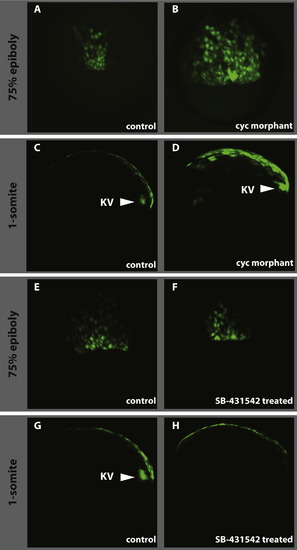
Effect of loss of Nodal signalling on dEVL and Kupffer’s vesicle formation. As compared to control (A and E), the cyc morpholino injections result in the expansion of the dorsal GFP domain (B) whereas the drug treatment doesn’t have any effect on dEVL (F). As in wild type (C) and control treated (G), Kupffer’s vesicle forms in cyc morphant (D, KV and arrowhead) where the vesicle is absent in SB-431542 treated embryos (H). A, B, E and F dorsal view at 60–70% epiboly. C, D, G, and H lateral view at 1-somite stage anterior is to the left. |
|
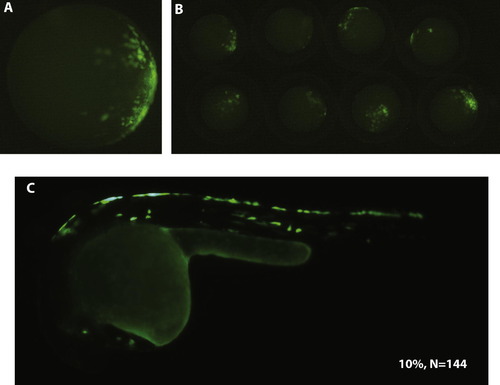
An internal 400bp promoter is sufficient to drive specific transgene expression in the dorsal EVL and dorsal periderm. A single embryo (A) and a group of 8 embryos (B) at 40% epibody show GFP expression in the presumptive dorsal site. At 24hpf, the larva shows GFP expression in the dorsal periderm (C). Transient expression analysis revealed that 10% of the injected embryos (N = 144) show specific GFP expression in the dorsal EVL and dorsal periderm. |
|
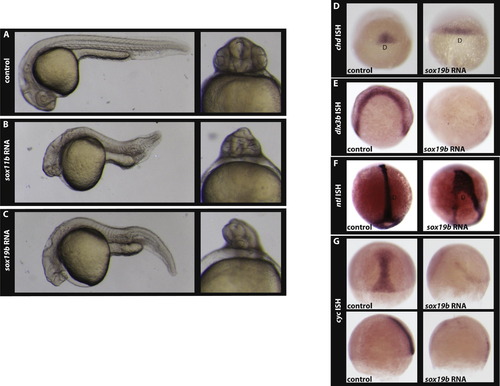
Over-expression of sox11b and sox19b causes a cyclopia phenotype. Control embryos (A), and embryos over-expressing sox11b (B), and sox19b (C) at 24hpf. In situ hybridisation analysis indicates that as compared to control embryos the chordin expression domain is expanded in sox19b injected embryos (D). In situ hybridisation (E) for dlx3b reveals that convergent extension is perturbed in embryos injected with sox19b mRNA. As a consequence, the formation of axial mesoderm is altered in sox19b mRNA injected embryos as visualised by ntl expression (F). As compared to the control, cyc expression is reduced (G) in embryos over-expressing sox19b at the 75% epiboly stage (upper panel, dorsal view; bottom panel, lateral view). D- dorsal in D and F. |
|
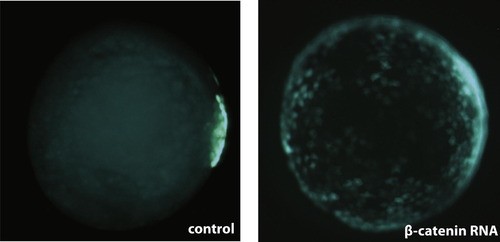
Over-expression of Β-catenin leads to an expansion of the dEVL in Tg(miniCrestin:Gal4, UAS:GFP). |
|
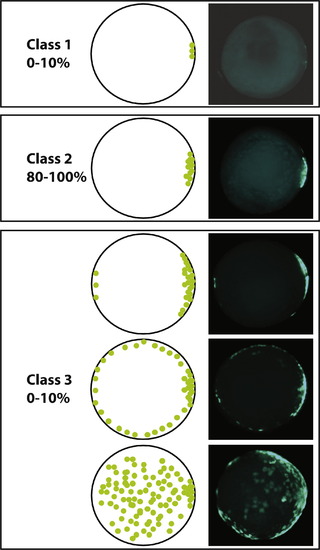
Classification of transgene expression. Variations in the transgene expression in an outcross were classified at the shield stage. Embryos with less than 20 GFP positive cells restricted to the dorsal domain were classified as Class 1. Embryos with more than 20 GFP positive cells localized in the dorsal hemisphere were classified as Class 2. Embryos with GFP positive cells expanding to the ventral hemisphere were classified as C3. |
Reprinted from Mechanisms of Development, 129(1-4), Chen, Y.Y., Harris, M.P., Levesque, M.P., Nüsslein-Volhard, C., and Sonawane, M., Heterogeneity across the dorso-ventral axis in zebrafish EVL is regulated by a novel module consisting of sox, snail1a and max genes, 13-23, Copyright (2012) with permission from Elsevier. Full text @ Mech. Dev.