- Title
-
Dermal morphogenesis controls lateral line patterning during postembryonic development of teleost fish
- Authors
- Wada, H., Ghysen, A., Satou, C., Higashijima, S.I., Kawakami, K., Hamaguchi, S., and Sakaizumi, M.
- Source
- Full text @ Dev. Biol.
|
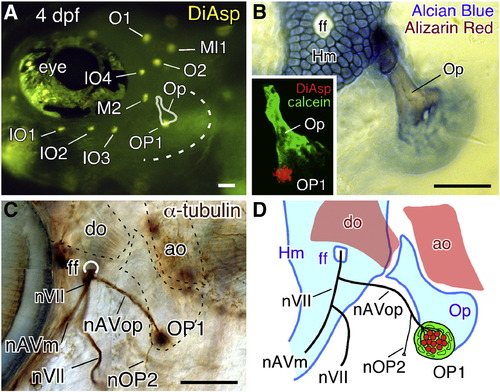
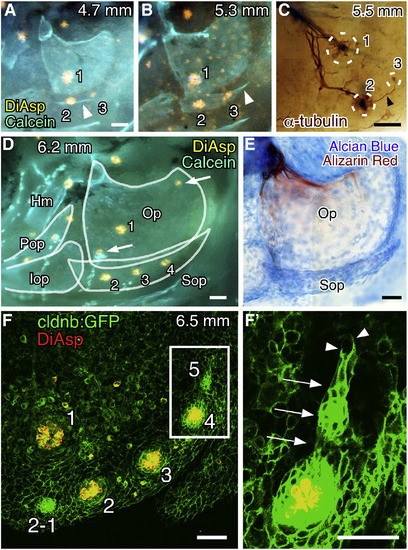
Opercular lateral line system in zebrafish embryos at 4 dpf. (A) Distribution of cranial neuromasts labeled with DiAsp, showing the outlined opercle (Op) and the operculum indicated by a dotted line. (B) Skeletal elements are visualized by Alcian blue and Alizarin red staining. Inset shows a confocal image of the operculum doubly stained with DiAsp and Calcein. (C) Staining with anti-acetylated tubulin antibody reveals the nerve projection. The opercle and its associated muscles are outlined by dotted lines. (D) Schematic drawing of the opercular structures. Hm, hyomandibular; ff, facial nerve foramen; do, dilator operculi; ao, adductor operculi; nVII, facial nerve; nAVm, mandibular ramus of the anteroventral lateral line nerve; nAVop, opercular ramus of the anteroventral lateral line nerve; nOP2, ramus projecting to the developing OP2. Lateral views; anterior is to the left. Scale bars: 50 μm. |
|
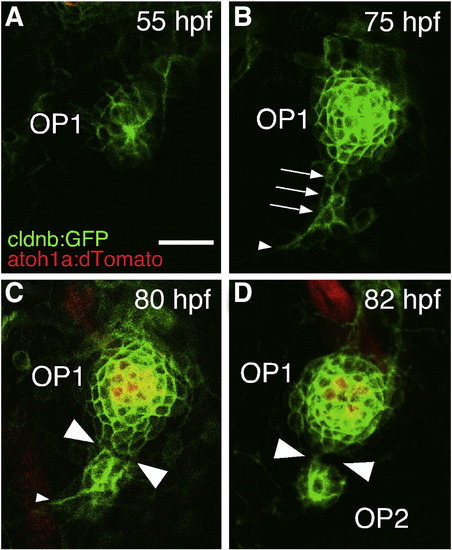
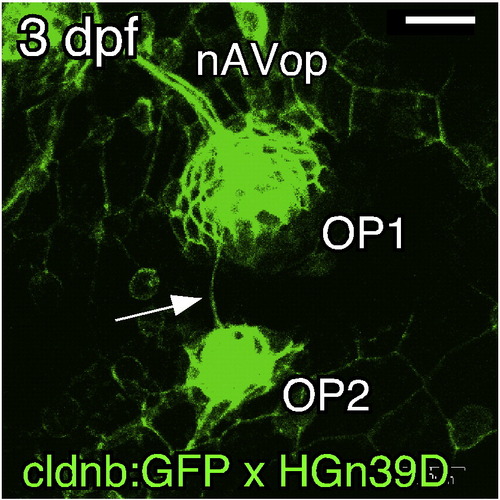
OP1 gives rise to OP2 by a budding process. (A–D) Confocal images of embryos doubly transgenic for cldnb:gfp and atoh1a:dtomato. The stages of the embryos are indicated in each panel, but the time of OP2 formation varied considerably among individuals (see text). Arrows indicate the budding structure; small arrowheads indicate leading processes; large arrowheads indicate the cleavage site. Lateral views; anterior is to the left. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
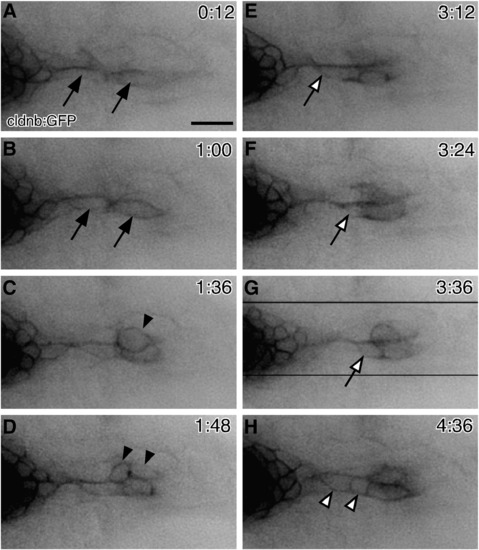
Behaviors of neuromast cells during a budding process. (A–H) Time-lapse observation of 3-dpf cldnb:gfp transgenic embryo. Images were selected from the original movie (Supplemental Movie S1). (A, B) Two elongated cells are extending from OP1 (indicated by arrows). (C, D) One of these cells becomes round shaped (C, arrowhead) and undergoes a cell division at a distal site (D, arrowheads). (E–G) A cell migrating distally along the elongated cells is indicated by open arrows. (H) Subsequently, cells are recruited proximally (open arrowheads). Time after the start of recording is indicated in each panel. The direction of budding is shown to the right. Scale bar: 20 μm. EXPRESSION / LABELING:
|
|
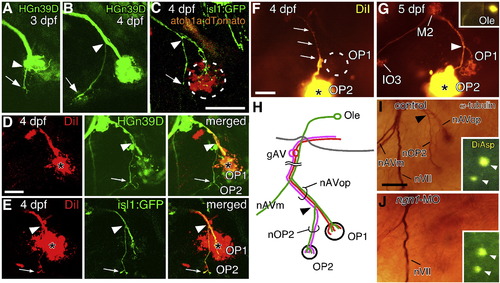
Projection of the lateral line nerve to the opercular neuromasts. HGn39D embryos at 3 dpf (A) and 4 dpf (B). Arrows indicate the sensory axon(s) extending to OP2 (nOP2); arrowheads indicate the point where nOP2 leaves the nAVop. (C) isl1:gfp and atoh1a:dtomato double-transgenic embryo at 4 dpf. (D, E) DiI injection in OP1, in HGn39D (D) and isl1:gfp (E) embryos at 4 dpf, reveals only efferent axons to OP2. Asterisks indicate the sites of DiI injection. Panels A to E are confocal images. (F, G) OP2 injected with DiI at 4 dpf (F) and 5 dpf (G). The position of OP1 is indicated by a dotted line in F. Several neuromasts (OP1, M2, and IO3) are labeled at 5 dpf (G). Inset in G indicates an octavolateralis efferent (Ole) neuron in the hindbrain. (H) Schematic drawing of the opercular lateral line projection. gAV, anteroventral lateral line ganglion. (I, J) Neuromast budding occurs normally in ngn1-MO injected embryos (J) as compared with control embryos (I). Insets show images of the same embryos stained with DiAsp before they were subjected to the immunohistochemistry. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
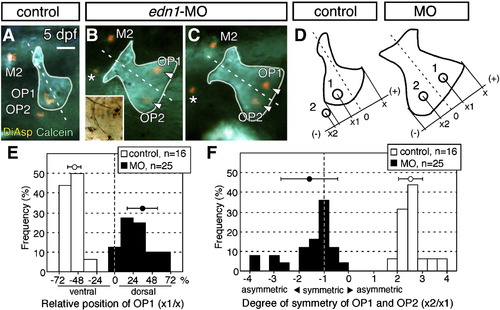
Deficient neuromast patterning in endothelin 1-knockdown embryos. (A) Embryo doubly stained with DiAsp and Calcein demonstrate the position of the neuromasts at 5 dpf. (B, C) Opercle and neuromasts in embryos injected with edn1-MO. The ossified region of the opercle is outlined, and the opercle midline is indicated by the dotted line. Asterisks indicate unidentified neuromasts appearing ectopically. Inset in B shows the same embryo immunostained for acetylated α-tubulin. (D) Schematic drawing for quantification of the neuromast distribution. Positions of the dorsal edge of the opercle (x), OP1 (x1) and OP2 (x2) are measured relative to the line of opercle symmetry (0). Positive or negative values define positions dorsal or ventral to the midline, respectively. (E) Position of OP1 relative to the opercle in edn1-MO larvae. The relative position of OP1 is defined as the ratio of x1/x (expressed as %). (F) Symmetrical distribution of the neuromasts with respect to the midline of the opercle in the morphant embryos. The degree of symmetry is defined as the ratio of x2/x1. When OP1 and OP2 are located symmetrically with respect to the midline, the degree of symmetry equals - 1. Mean ± SEM are indicated respectively by the circles and bars on top of the graph. Scale bar: 50 μm. PHENOTYPE:
|
|
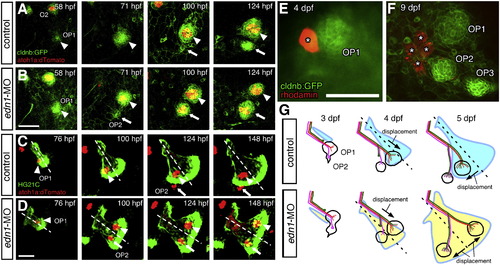
Morphogenesis of the opercle correlates with neuromast patterning. (A–D) Time-course observations of control (A, C) and edn1-MO injected (B, D) embryos doubly transgenic for atoh1a:dtomato and cldnb:gfp (A, B), or HG21C (C, D). In morphant embryos, OP2 forms earlier than in control embryos (A, B). In control embryos, both OP1 and OP2 are located ventral to the opercle midline (C). In contrast, in morphant embryos, OP1 is located on the dorsal half of the opercle and moves away from OP2, which is located on the ventral opercle (D). Arrowheads and arrows indicate OP1 and OP2, respectively. (E, F) Single peridermal cell was labeled with rhodomine-dextrane and traced its fate, showing proliferation of peridermal cells relative to the neuromasts. (G) Schematic drawings of the developing opercular lateral line system in control and morphant embryo. The stage of the embryo is indicated in each panel. Scale bars: 50 μm. |
|
Development of the neuromast series on the subopercle. (A, B) Formation of OP3 along the extending subopercle visualized by staining with DiAsp and Calcein. Arrowhead indicates the subopercle. Body length of the larva is indicated in each panel. (C) Embryo immunostained for acetylated α-tubulin showing OP3 innervated by a nerve extending from OP2 (arrowhead). Neuromast positions are indicated by dotted circles. (D) Formation of OP4. Opercular bones are outlined. Hm, hyomandibular; Pop, preopercle; Iop, interopercle. Arrows indicate unidentified neuromasts in irregular positions on the opercle. (E) The same larva shown in D was stained with Alcian blue and Alizarin red. (F) Confocal image of a cldnb:gfp larva stained with DiAsp. (F′) Higher magnification of the boxed region in F, showing a budding structure (arrows). Arrowheads indicate the leading processes. Scale bars: 50 μm. EXPRESSION / LABELING:
|
|
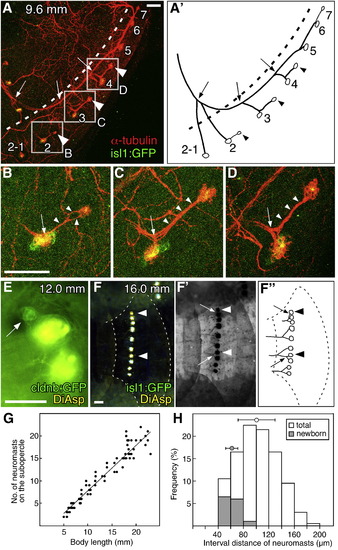
Development of the pedigree-like architecture of the opercular lateral line. (A) isl1:gfp juvenile fish stained with anti-GFP and anti-acetylated α-tubulin antibodies. (A′) Schematic drawing of the lateral line architecture. Arrowheads indicate newborn neuromasts; arrows indicate the bifurcation points left behind by moving neuromasts; dotted lines indicate the boundary between opercle and subopercle. (B–D) Higher magnifications of the boxed regions in A. Arrowheads indicate the nerve extending from the mother neuromasts; arrows indicate bifurcations of the nerve. (E) Stitch formation on a body scale. Arrow indicates the budding structure. (F) isl1:gfp juvenile fish stained with DiAsp. Arrowheads indicate newborn neuromasts. (F′) The same image was converted to negative and monochrome to show the axonal trajectories retrogradely labeled with DiAsp. (F″) Schematic drawing of the innervation pattern in F′. Arrows indicate the nerve innervating the newborn neuromasts. (G) The number of DiAsp-positive neuromasts on the subopercle increases during postembryonic development. (H) Quantification of interval length between adjacent DiAsp-positive neuromasts. Intervals between newly born neuromasts and their mother neuromasts are indicated by gray bars. Mean ± SEM are indicated respectively by the circles and bars on top of the graph. Scale bars: 50 μm. EXPRESSION / LABELING:
|
|
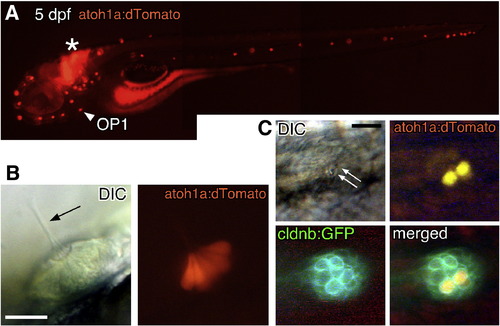
atoh1a:dtomato expresses dTomato in the differentiated hair cells. (A) atoh1a:dtomato embryo at 5 dpf. dTomato is expressed in all neuromasts and some hindbrain interneurons (asterisk). Arrowhead indicates OP1. (B) Lateral view of a neuromast showing dTomato expressed in all or most hair cells. (C) Embryo doubly transgenic for atoh1a:dtomato and cldnb:gfp. A pair of hair cells (arrows indicate the sensory hairs) expresses dTomato. Scale bars: 50 μm. |
|
Sensory axons follow the budding structure. Single confocal image of a 3-dpf embryo doubly transgenic for cldnb:gfp and HGn39D. The OP1 and OP2 proneuromasts are connected by a sensory nerve (arrow). Scale bar: 20 μm. |
|
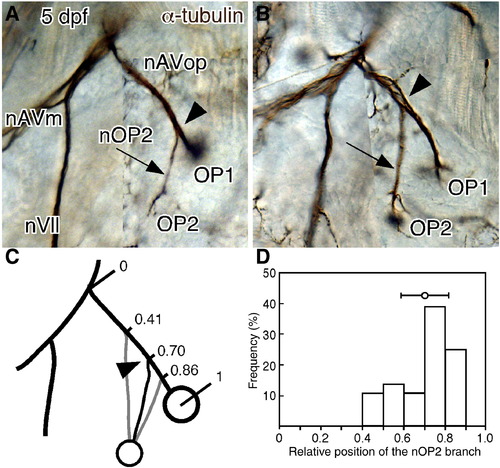
Variability in the OP1/OP2 bifurcation point. (A, B) Left and right sides of 5-dpf embryo immunostained for acetylated α-tubulin. The right side is mirror-imaged by Photoshop for comparison. The positions of the OP1/OP2 bifurcating point (indicated by arrowheads) are different between the two sides. (C, D) Distribution of bifurcation points. Mean ± SEM is indicated in the figure (n = 36). Scale bars: 50 μm. |
|
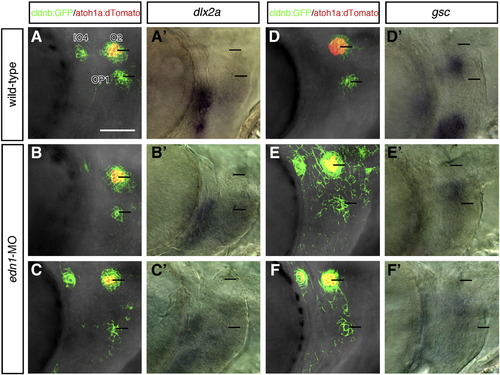
Reduced expression of dlx2 and gsc genes in edn1-knockdown embryos. Confocal images of control (A, D) and edn1-MO injected (B, C, E, F) embryos doubly transgenic for cldnb:gfp and atoh1a:dtomato at 52–54 hpf. Each embryo was fixed immediately after the observation and stained with the dlx2a (A′–C′) or gsc (D′–F′) RNA probes. The expression of both genes was severely reduced in morphant embryos, but the position of OP1 was not affected. Two morphant embryos are shown for each staining, which represent different severities of reduction in gene expression. Lines indicate positions of O2 and OP1. Scale bar: 50 μm. |
Reprinted from Developmental Biology, 340(2), Wada, H., Ghysen, A., Satou, C., Higashijima, S.I., Kawakami, K., Hamaguchi, S., and Sakaizumi, M., Dermal morphogenesis controls lateral line patterning during postembryonic development of teleost fish, 583-594, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.