- Title
-
Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia
- Authors
- Huang, P., and Schier, A.F.
- Source
- Full text @ Development
|
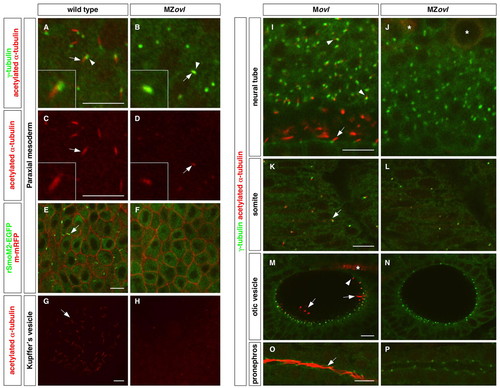
Zebrafish MZovl mutants lack cilia. Cilia were visualized by staining with acetylated α-tubulin antibody (red) (A-D,G-P) and basal bodies were visualized by staining with γ-tubulin antibody (green) (A,B,I-P). (A-D) The ciliary axoneme (arrows) and the basal body (arrowheads) are formed in cells of the paraxial mesoderm in wild-type embryos (A,C), whereas cilia (arrows) are absent in MZovl mutants (B,D). Note that domains of stabilized microtubules (arrows in B,D) can only be found co-localizing with the basal body (arrowhead in B) in MZovl mutants. C and D correspond to the red channel of A and B, respectively. Insets show magnified views of regions of interest. (E,F) Cilia (arrow) labeled by rSmoM2-EGFP (green) are present in wild-type embryos but not in MZovl mutants. The cell membrane was labeled with m-mRFP (red). (G-P) Cilia (arrows and arrowheads) are absent in Kupffer′s vesicle (G,H), the neural tube (I,J), somites (K,L), the otic vesicle (M,N), and the pronephric duct (O,P) in MZovl mutants as compared with control embryos. In the neural tube (I), both floor plate cilia (arrow) and cilia in neurons of the dorsal neural tube (arrowheads) are indicated. In the otic vesicle (M), both the longer tether cilia (arrows) and the short non-motile cilia (arrowhead) are indicated. Note that despite the absence of cilia, the basal body forms in MZovl mutants. Asterisks in J and M mark the staining of some neurons and axons, which also label with antibody to acetylated α-tubulin. Embryos are at the 10-somite stage (A-D), 1-somite stage (E,F), 8-somite stage (G,H) and at 24 hpf (I-P). A-H are dorsal views and I-P are lateral views. Scale bars: 10 μm. |
|
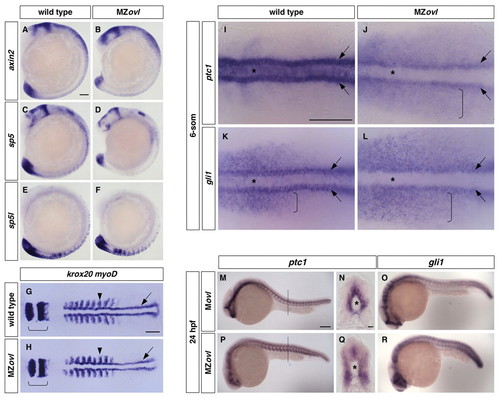
Characterization of MZovl mutants. (A-F)MZovl mutants (B,D,F) show similar expression of axin2 (A,B), sp5 (C,D) and sp5l (E,F) to wild-type embryos (A,C,E). (G,H)MZovl mutants (H) display similar anterior-posterior axis formation to wild-type embryos (G) as indicated by krox20 (rhombomeres 3 and 5, brackets) and myoD [somites (arrowheads) and adaxial cells (arrows)] expression. (I-L)MZovl mutants show reduced ptc1 and gli1 expression in the ventral neural plate (out of focus, asterisks) and adaxial muscle cells (arrows), and expanded expression in the paraxial mesoderm (brackets). (M-R)MZovl mutants (P-R) show reduced, but expanded, ptc1 and gli1 expression compared with control Movl embryos (M-O). N and Q are cross-sectional views of M and P (dashed lines indicate plane), respectively, with the notochord marked by an asterisk. In situ hybridization was carried out in embryos at the 15-somite stage (A-F), 9-somite stage (G,H), 6-somite stage (I-L) and at 24 hpf (M-R). A-F,M,O,P,R are lateral views and G-L are dorsal views. Scale bars: 100 μm in A-M,O,P,R; 10 μm in N,Q. |
|
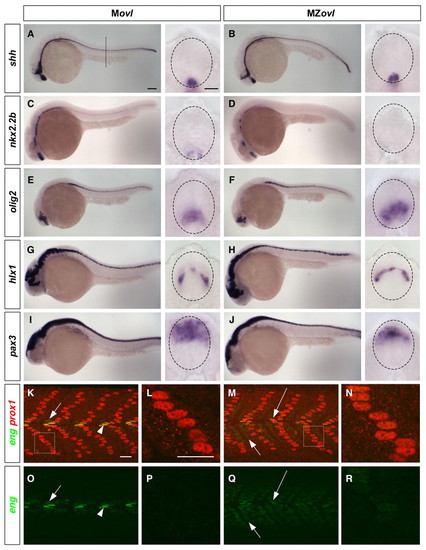
Neural and somite patterning in MZovl mutants. (A-J) Marker analysis of neural patterning. Movl control zebrafish embryos (A,C,E,G,I) and MZovl mutants (B,D,F,H,J) were stained at 24 hpf for the expression of shh (A,B), nkx2.2b (C,D), olig2 (E,F), hlx1 (G,H) and pax3 (I,J). Each panel contains a lateral view and a cross-sectional view (between somites 10 and 16; dashed line in A indicates plane) with the neural tube outlined (dashed lines). MZovl mutants show normal shh and hlx1 expression (B,H), an absence of nkx2.2b expression (D), expanded olig2 expression (F), and reduced pax3 expression (J) in the neural tube. (K-R) Marker analysis of somite patterning. Movl control embryos (K,L,O,P) and MZovl mutants (M,N,Q,R) were stained at 24 hpf with antibodies against eng (green) and prox1 (red). Muscle pioneers (prox1+, strong eng+, arrowheads in K,O) are replaced with prox1+, weak eng+ cells (long arrows in M,Q) in MZovl mutants. Medial fast fibers (prox1-, weak eng+) are expanded in MZovl mutants (short arrows in M,Q) as compared with Movl embryos (short arrows in K,O). Superficial slow fibers (prox1+) also express a low level of eng in MZovl mutants (N,R) as compared with Movl controls (L,P). K-N are merged confocal images with both green and red channels, and the corresponding green channel images are shown in O-R, respectively. L and N are higher magnification views of the boxed regions in K and M, respectively. Lateral views. Scale bars: 100 μm in lateral views in A-J; 10 μm in cross-sections in A-J; 20 μm in K-R. |
|
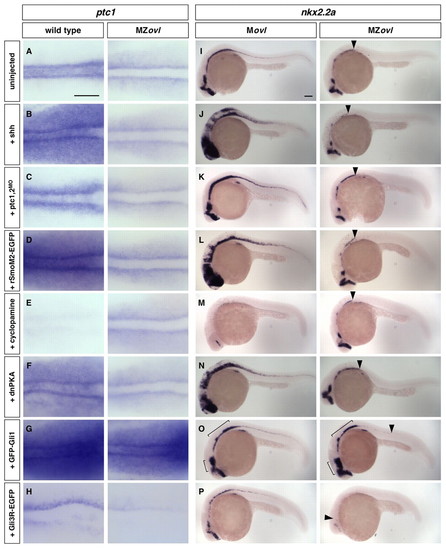
Epistasis analysis of cilia and the Hh signaling pathway. (A-P)MZovl and control zebrafish embryos were stained for the expression of ptc1 (A-H) and nkx2.2a (I-P) following different manipulations. MZovl embryos are insensitive to ectopic expression of shh mRNA (B,J), depletion of ptc1,2 using morpholinos (C,K), expression of rSmoM2-EGFP mRNA (D,L), treatment with cyclopamine at 100 μM (E,M), and expression of dnPKA mRNA (F,N). By contrast, expression of GFP-Gli1 (G,O) enhances, whereas expression of Gli3R-EGFP (H,P) reduces, ptc1 and nkx2.2a expression. Arrowheads in I-P indicate the posterior extent of nkx2.2a expression in MZovl mutants. Brackets in O indicate regions with expanded nkx2.2a expression. A-H are dorsal views of the presomitic mesoderm of 5-somite stage embryos, and I-P are lateral views of 24 hpf embryos. Scale bars: 100 μm. |
|
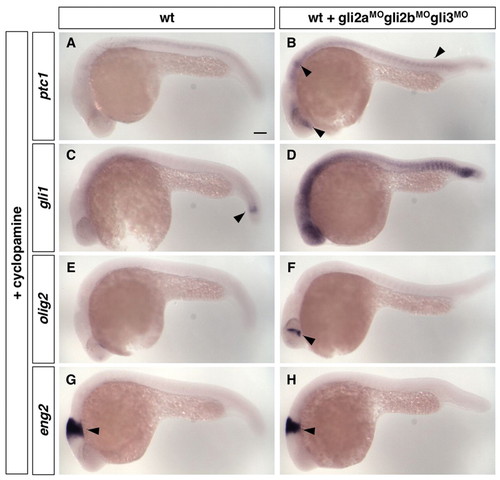
Loss of Gli repressors in the absence of Hh signaling is not sufficient to activate the Hh pathway. Wild-type control zebrafish embryos (A,C,E,G) and wild-type embryos injected with a cocktail of gli2a, gli2b and gli3 morpholinos (B,D,F,H) were treated with cyclopamine from 50% epiboly to 24 hpf, and stained for the expression of ptc1 (A,B), gli1 (C,D), olig2 (E,F) and eng2 (G,H). (A-D) Embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show a slight upregulation of ptc1 expression (arrowheads in B) and substantial upregulation of gli1 expression (D). Note that gli1 is expressed at a low level in the absence of Hh signaling (arrowhead in C). (E,F) Compared with control embryos (E), embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show a small patch of olig2 expression in the brain (arrowhead in F), but display no olig2 expression in the neural tube similar to controls. (G,H) Embryos treated with cyclopamine, gli2aMO, gli2bMO and gli3MO show no eng2 expression in somites similar to controls. Note that expression of eng2 in the midbrain-hindbrain boundary (arrowheads) does not require Hh signaling. Lateral views. Scale bar: 100 μm. |
|
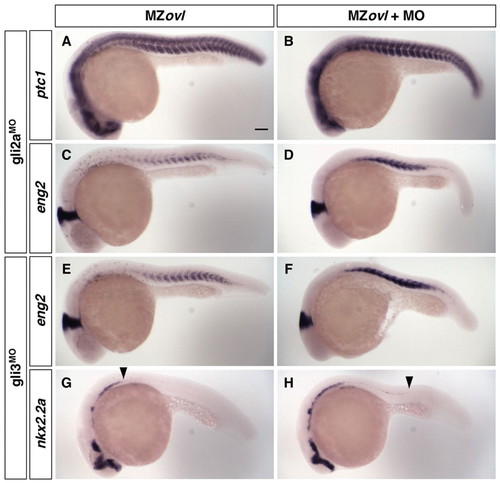
Gli repressor activity is present in MZovl mutants. MZovl control zebrafish embryos (A,C,E,G) and morpholino-injected MZovl mutants (B,D,F,H) were stained at 24 hpf for the expression of ptc1 (A,B), eng2 (C-F) and nkx2.2a (G,H). (A-D) gli2aMO-treated MZovl embryos show enhanced ptc1 and eng2 expression as compared with uninjected MZovl controls. (E-H) gli3MO-treated MZovl embryos show enhanced eng2 expression and partially rescued nkx2.2a expression in the neural tube compared with uninjected MZovl controls. Arrowheads in G and H indicate the posterior extent of nkx2.2a expression in the neural tube. Lateral views. Scale bar: 100 μm. |
|
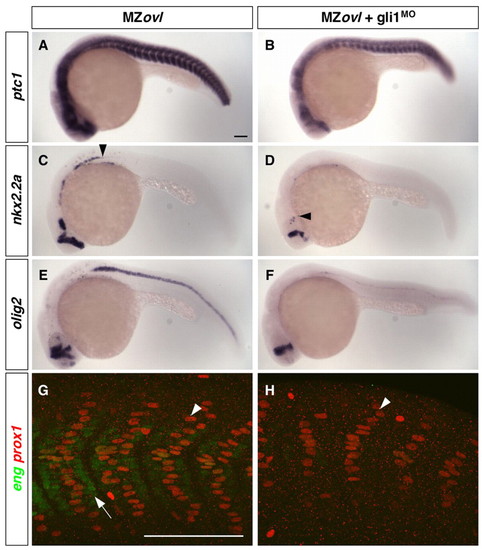
Expanded Hh pathway activity in MZovl mutants is dependent on Gli1 activity. (A-H) Uninjected MZovl contrls (A,C,E,G) and gli1MO-injected MZovl mutants (B,D,F,H) were stained at 24 hpf for the expression of ptc1 (A,B), nkx2.2a (C,D), olig2 (E,F), eng (green) and prox1 (red) (G,H). Note that gli1MO-treated MZovl embryos show reduced expression of ptc1 (B), nkx2.2a (D) and olig2 (F) as compared with corresponding untreated MZovl control embryos (A,C,E). Arrowheads in C and D indicate the posterior extent of nkx2.2a expression. In somites of gli1MO-treated MZovl embryos (H), medial fast fibers (prox1-, weak eng+, arrow in G) are abolished, and superficial slow fibers (prox1+, arrowheads in G,H) are substantially reduced in number. Lateral views. Scale bars: 100 μm. |
|
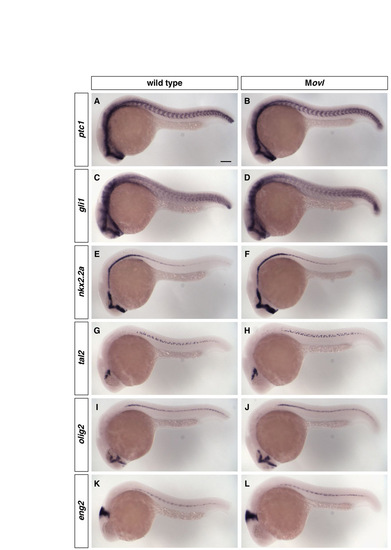
Movl embryos are indistinguishable from wild-type embryos at 24 hpf. (A-L) Wild-type embryos (a,c,e,g,i,k) and Movl embryos (b,d,f,h,j,l) at 24 hpf were stained for the expression of ptc1 (A,B), gli1 (c,d), nkx2.2a (e,f), tal2 (g,h), olig2 (i,j) and eng2 (k,l). All markers show similar staining patterns between wild-type and Movl embryos. Lateral views. Scale bar: 100 μm. |
|
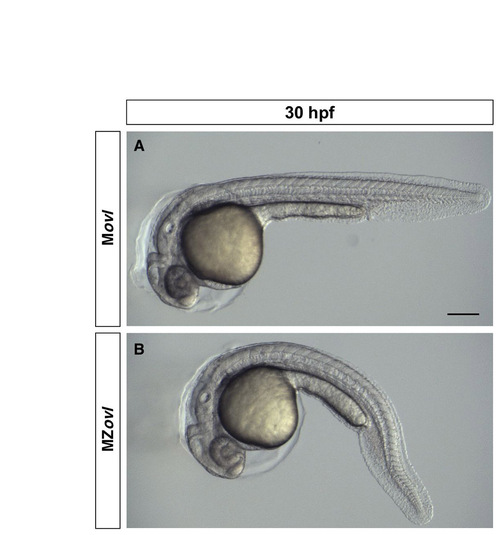
MZovl mutants have relatively normal morphology. Bright-field images of Movl control embryo (A) and MZovl mutant (B) at 30 hpf. Note that other than the body curvature phenotype, MZovl mutants have relatively normal overall morphology. Scale bar: 100 μm. |
|
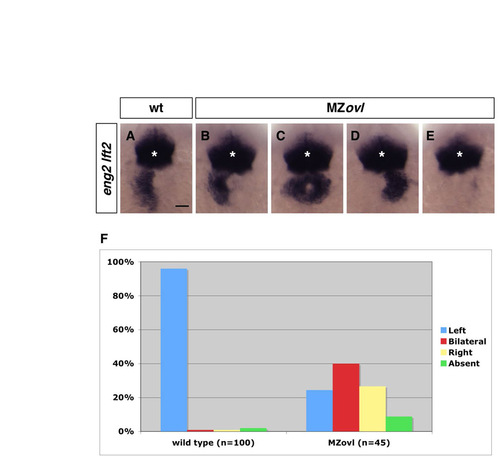
MZovl mutants have randomized left-right patterning. (a-e) MZovl mutants (b-e) display randomized lft2 expression compared with wild-type embryos (a). Symmetric eng2 expression (asterisks) in the midbrain-hindbrain boundary remains normal in MZovl mutants and is used as an internal landmark to compare lft2 expression between embryos. Examples of MZovl mutants with lft2 expression on the left (b), right (d), or both sides (c), or with no lft2 expression (e), are shown. Dorsal views of 22-somite stage embryos. Scale bar: 50 μm. (f) Quantification of left-right defects in MZovl mutants. Of wild-type embryos, 96% (n=100) show lft2 expression on the left side, whereas MZovl mutants (n=45) show randomized lft2 expression: 24% on the left side (B), 27% on the right side (D), 40% bilaterally (C), and 9% with no expression (E). |
|
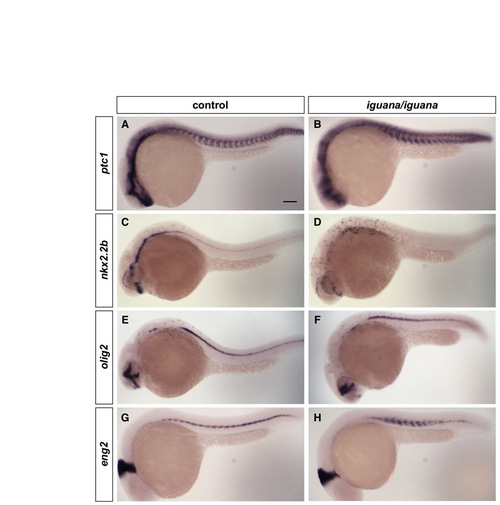
Neural and somite patterning in iguana mutants. (A-H) Control embryos (igu/+ or +/+) (a,c,e,g) and igu/igu mutants (b,d,f,h) at 24 hpf were stained for the expression of ptc1 (A,B), nkx2.2b (c,d), olig2 (e,f) and eng2 (g,h). igu/igu mutants show dampened, but expanded, ptc1 expression (B). Accordingly, nkx2.2b expression in the neural tube is lost in igu/igu mutants (D), whereas olig2 expression in the neural tube (F) and eng2 expression in the somite (H) are expanded. Lateral views. Scale bar: 100 μm. |
|
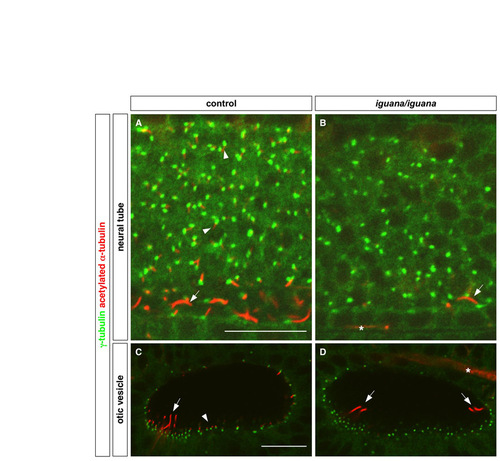
iguana mutants lack primary cilia. Cilia were visualized by staining with acetylated α-tubulin antibody (red), and basal bodies were visualized by staining with γ-tubulin antibody (green). (A,B) Primary cilia in the neural tube (arrowheads in A) are absent in igu/igu mutants (B), whereas the longer floor plate cilia (arrows) are reduced in number in igu/igu mutants as compared with control embryos (igu/+ or +/+) (A). (C,D) igu/igu mutants lack short non-motile cilia (arrowhead in C) and have a reduced number of the longer tether cilia (arrows) in the otic vesicle. Note that the cluster of tether cilia at the opposite end of the otic vesicle is out of focus in the control embryo (C). Asterisks in B and D mark the staining of axons, which also label with antibody to acetylated α-tubulin. Lateral views at 24 hpf. Scale bars: 20 μm. |
|
Epistasis analysis of cilia and Hh signaling in somite patterning. (A-J) Movl control embryos (a,c,e,g,i) and MZovl mutants (b,d,f,h,j) were stained for the expression of eng (green) and prox1 (red) following different manipulations. Ectopic expression of shh mRNA (C), depletion of ptc1,2 using morpholinos (E), and expression of dnPKA mRNA (I) in Movl embryos induce ectopic muscle pioneer cells (prox1+, strong eng+, arrows) and superficial slow fibers (prox1+) as compared with uninjected controls (a), whereas treatment with cyclopamine at 100 μM (g) blocks the formation of most muscle pioneers, superficial slow fibers and medial fast fibers (prox1-, weak eng+, arrowhead in A). By contrast, MZovl embryos are insensitive to these manipulations and are indistinguishable from untreated MZovl embryos, in which medial fast fibers (arrowheads) are expanded (b,d,f,h,j). Lateral views at 24 hpf. Scale bar: 100 μm. |