- Title
-
The zebrafish vitronectin receptor: Characterization of integrin alphaV and beta3 expression patterns in early vertebrate development
- Authors
- Ablooglu, A.J., Kang, J., Handin, R.I., Traver, D., and Shattil, S.J.
- Source
- Full text @ Dev. Dyn.
|
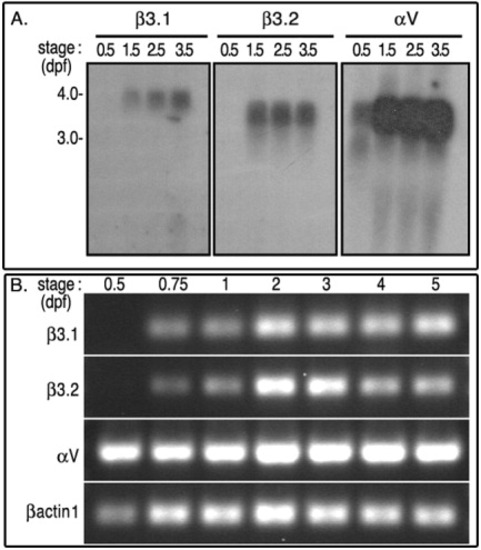
mRNA expression profiles of αV, β3.1, and β3.2. A: Approximately 15 μgr total RNA extracted from 0.5, 1.5, 2.5, and 3.5 dpf zebrafish larva, was resolved in denaturing agarose gel, and processed for Northern blot analysis. B: The expression of these integrins analyzed by semi-quantitative RT-PCR. Total RNAs were extracted from 3-5 somite (0.5 dpf), 16-18 somites (0.75 dpf), 1 dpf, 2 dpf embryos, and later from 3 dpf, 4 dpf, and 5.5 dpf swimming larva. RT-PCR reactions were done with sequence-specific primers against αV (172 bp), β3.1 (312 bp), and β3.2 (385 bp). RT-PCR reactions were also monitored with amplification of zebrafish βactin1 fragment (172 bp), which was used as a positive loading control. EXPRESSION / LABELING:
|
|
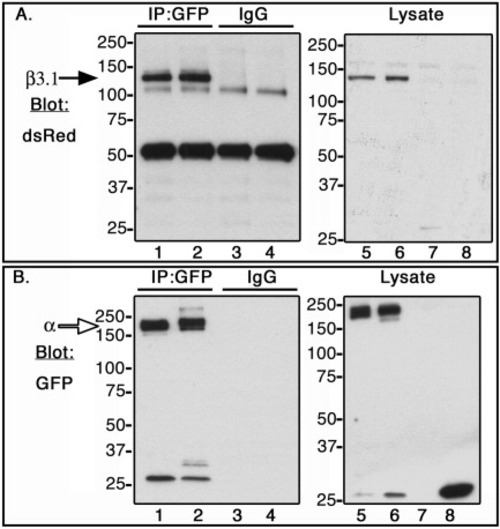
Co-immunoprecipitation of zebrafish αV, αIIb, and β3.1 subunit in CHO cells. C-terminal α subunits fused to GFP (open arrow, ∼180 kDa), or β3.1 subunit fused to DsRed monomer (black arrow, ∼130 kDa) were expressed in CHO cells. Lysates were incubated with anti-GFP antibody (IP:GFP, lanes 1 and 2), and with isotype control antibody, rabbit IgG (IgG, lanes 3 and 4). Co-immunoprecipitated samples (lanes 1-4), and whole cell lysates (lanes 5-8) were Western blotted with dsRed antibody (A) or GFP antibody (B). CHO cells were transfected in the following order: Lanes 1, 3, and 6, αIIb-GFP + β3.1-dsRed; Lanes 2, 4, and 5, αV-GFP + β3.1-dsRed; Lane 7, DsRed monomer; Lane 8, GFP. |
|
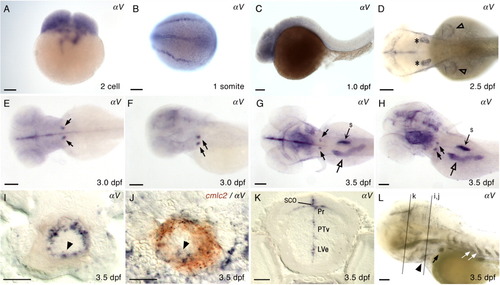
Expression of αV in wild type zebrafish. Embryonic and larval stages: (A) 2-cell; (B) 1 somite; (C) 1 dpf; (D) 2.5 dpf; (E,F) 3.0 dpf; (G-L) 3.5 dpf. Marked expression fields: ears (asterisk), AER of pectoral fins (open arrowhead), bilateral expression in the deep posterior, ventral pharyngeal region (black arrows), intestine (open arrow), and swim bladder (s). Transverse sections of a 3.5-dpf larva at the level of heart (I, J), and at the level of eye (K). I: αV expression in the ventricle of embryonic heart tube (black arrowhead). J: Double ISH larva, cmlc2 (red), αV (dark purple). K: αV expression in brain: LVe, lateral recess ventricle of hypothalamus; Pr, pretectum; PTv, ventral part of posterior tuberculum; SCO, subcommissural organ. L: Longer staining of 3.5 dpf larva: Intersegmental vessels (white arrow); heart ventricle (black arrowhead). Relative locations for sections in I, J, and K are indicated with gray lines in L. Lateral views of embryos, anterior to the left, are shown in C, F, H, and L. Larva in F and H were slightly tilted. Dorsal views of the embryos, anterior to the left, are shown in B, D, E, and G. Scale bars = 100 μm in A-H and L, and 20 μm in I-K. EXPRESSION / LABELING:
|
|
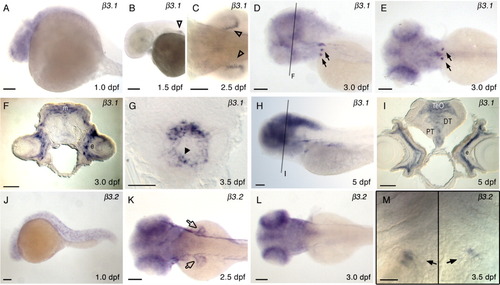
Expression of β3.1 (A-I), and β3.2 (J-M) in wild type zebrafish. Embryonic and larval stages: (A, J) 1 dpf; (B) 1.5 dpf; (C, K) 2.5 dpf; (D, E, F, L) 3.0 dpf; (G, M) 3.5 dpf; (H, I) 5 dpf. Marked expression fields are similar to Figure 3, except in K where β3.2 expression in pectoral fins is marked with open arrow. Transverse sections at the level of eye (F, I), and at the level of heart (G). F: β3.1 expression in 3.0 dpf zebrafish brain. m, medial tectal proliferation zone; e, eye. G: At 3.5 dpf β3.1 expression is present in the ventricle of embryonic heart tube (black arrowhead). I: β3.1 expression in 5.0 dpf zebrafish brain. DT, dorsal thalamus; e, eye; PT, posterior tuberculum; TeO, tectum opticum. Lateral views of embryos, anterior to the left, are shown in A, B, D, H, and J. Larva in D was slightly tilted to visualize bilateral expression in the deep posterior, ventral pharyngeal region. M: Two images of the same specimen, divided by a black line, at slightly different focal planes were taken to show β3.2 expression fields in the left and right halves of the deep posterior, ventral pharyngeal region: Ventral view of the specimen, anterior to the top, centered at the fifth ceratobranchial. Dorsal views of the embryos are shown in C, E, K, and L. Scale bars = 100 μm in A-E, H, J-L, 20 μm in F, G, I, and M. |
|
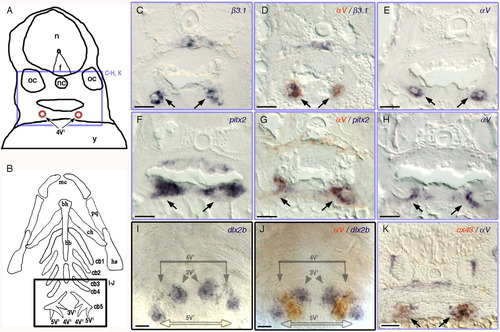
A-K:αV and β3.1 are expressed in the dental epithelium of the pharyngeal tooth. A: Camera lucida drawing of 3.5 dpf larval transverse sections. Purple box represents the location of the images in the transverse sections in C-H, and K. 4V1, first forming tooth; f, floor plate; n, neural tube; nc, notochord; oc, otic capsule; y, yolk. B: Diagram of the pharyngeal cartilages in 3.5 dpf young larva, ventral view. Black box representing the location of the images in I and J. 3V1/5V1, second forming teeth pair; bb, basibranchials; bh, basihyal; cb, ceratobranchials; ch, ceratohyal; hs, hyosymplectic; mc, Meckel's catilage; pq, platoquadrate. Expression of β3.1 (C,D), αV (D,E, G,H, J,K), pitx2 (F,G), dlx2b (I,J), cx43 (K) in wild type zebrafish. Double ISH samples: (D) αV (orange-red), β3.1 (dark purple); (G) αV (orange-red), pitx2 (dark purple); (J) αV (orange-red), and dlx2b (dark purple); (K) αV (dark purple), and cx43 (orange-red). Scale bars = 20 μm. EXPRESSION / LABELING:
|
|
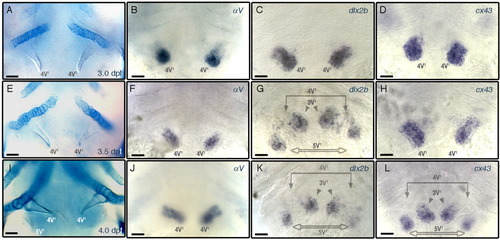
Spatial and temporal expression patterns of αV, dlx2b, and cx43. A-L: Ventral views of specimens centered at the fifth ceratobranchial, focused at the level of teeth, anterior to the top and oriented as in Figure 5B. A, E, I: Alcian blue stained cartilages. Whole mount ISH specimens probed with αV (B, F, J), dlx2b (C, G, K), and cx43 (D, H, L). Larval stages are identical for each row: 3.0 dpf (A-D); 3.5 dpf (E-H); 4.0 dpf (I-L). Scale bars = 20 μm. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|