- Title
-
Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects in choker mutants
- Authors
- Svetic, V., Hollway, G.E., Elworthy, S., Chipperfield, T.R., Davison, C., Adams, R.J., Eisen, J.S., Ingham, P.W., Currie, P.D., and Kelsh, R.N.
- Source
- Full text @ Development
|
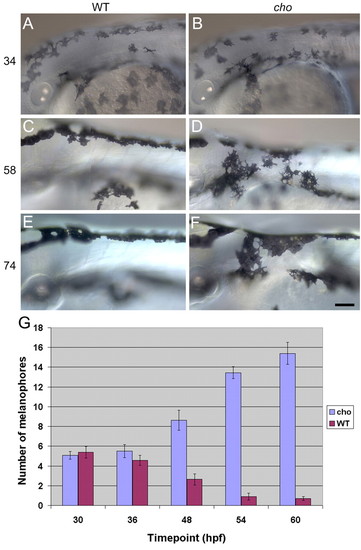
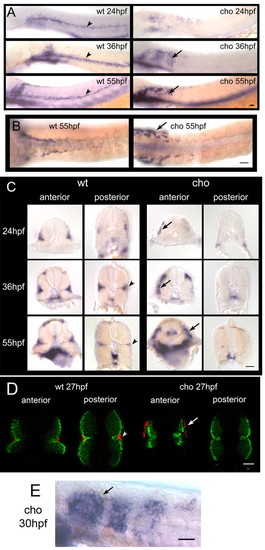
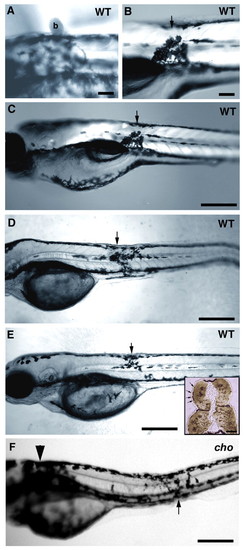
Timecourse of anterior trunk pigment pattern formation in WT and cho mutants during period of collar formation. Lateral views of WT (A,C,E) and cho mutant (B,D,F) embryos are shown. Melanophores were seen on the lateral pathway in cho mutants and WT siblings at 34 hpf (A,B), and were thereafter absent from WT, but accumulated steadily in cho mutants (C-F). Note too the change in collar melanophore morphology between 58 hpf, when their stellate morphology correlated with migratory activity, and 74 hpf, when their more flattened shape reflects a more static phase. Scale bar: 50 μ;m. (G) Total number (means±s.e.) of melanophores on lateral pathway of somites 1-5 throughout collar formation. PHENOTYPE:
|
|
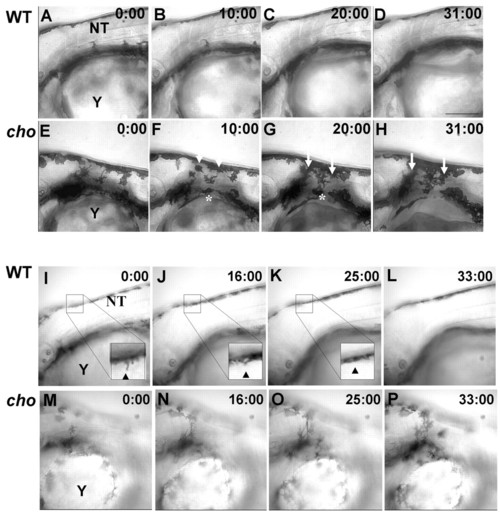
Timelapse microscopy of 48 hpf WT and cho mutants. (A-H) Images from different focal planes combined by projection revealed that WT melanophores (A-D) never entered the anterior trunk lateral pathway. By contrast, cho mutant melanophores (E-H) invaded the collar, both as single cells from the ventral stripe (asterisk) and as a sheet from the dorsal stripe (arrows). (I-P) Images from single focal planes clarify movements of individual cells. WT melanophores (I-L) extend processes into the anterior trunk (arrowhead, I) but these retract (arrow, arrowhead in J,K), whilst cho mutant melanophores (M-P) invade the collar, usually remaining ectopically. NT, neural tube; Y, yolk. Scale bar: 150 μm. PHENOTYPE:
|
|
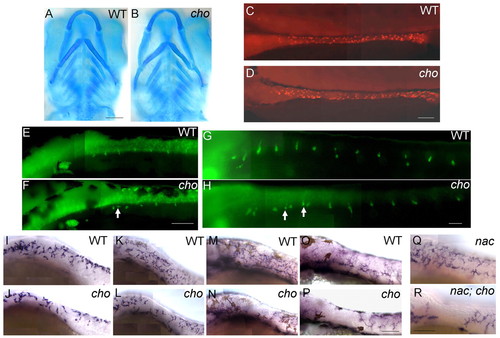
NC derivatives of lateral, but not medial, migration pathway are defective in cho mutants. (A,B) Alcian Blue staining of craniofacial cartilage in 5 dpf WT (A) and cho mutant (B) embryos. (C-H) Anti-Hu detection of enteric neurons at 3 dpf in WT (C) and cho mutant (D) embryos, and of DRG sensory neurons at 48 hpf (E,F) and 5 dpf (G,H) in WT (E,G) and cho mutant (F,H) embryos. As reported previously in WT embryos (An et al., 2002), we observed some ectopic sensory neurons at both 48 hpf and 5 dpf (arrows, F,H; see also Table 1). (I-P) In situ hybridisation with GTP-cyclohydrolase (gch) on WT (I,K,M,O) and cho mutants (J,L,N,P) at 24 hpf (I,J), 30 hpf (K,L), 36 hpf (M,N) and 48 hpf (O,P). (Q,R) 48 hpf. cho mutant collar xanthophore defects are independent of melanophores. In situ hybridisation with gch showed that whilst nac mutants had a WT xanthoblast pattern throughout the trunk (Q), cho;nac double mutants still displayed loss of xanthoblasts from the collar region (R). Embryos are shown in lateral view, anterior to the left; except for A,B which are ventral views, anterior up. Scale bars: A,B, 50 μm; C,D,G,H, 75 μm; E,F, 100 μm; I-P, 150 μm; Q,R, 175 μm. |
|
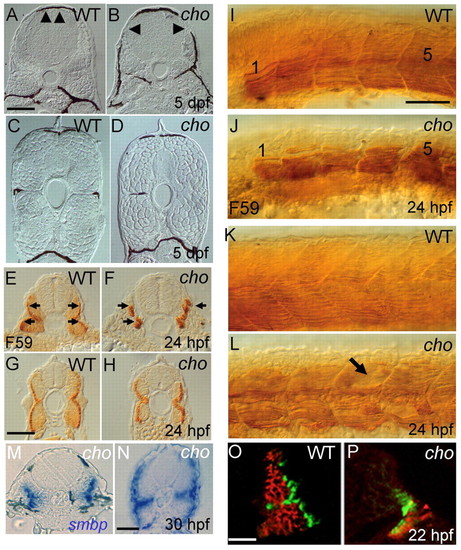
Somite defects in cho mutants. (A-D) Transverse sections of 5 dpf embryos reveal defective muscle block extension in anterior (arrowheads, A,B), but not posterior (C,D), trunk of cho mutants (B,D) as compared with WT (A,C). cho mutants typically lack melanophores and horizontal myoseptum; exceptionally, as here (left side), a single melanophore may be present, but in an abnormal position. (E-H) Sections of anterior (E,F) and posterior (G,H) trunk of 24 hpf WT (E,G) and cho mutant (F,H) embryos labelled with F59 antibody to show slow muscle fibres (arrows in E,F). (I-L) Lateral view of anterior (I,J; somites 1 and 5 indicated) and posterior (K,L) trunk of 24 hpf WT (I,K) and mutant (J,L) embryos labelled with F59 antibody; note the disorganised slow muscle fibres and apparent holes in fibre pattern (arrow) in cho mutant. (M,N) Transverse sections of anterior (M) and posterior (N) trunk of 30 hpf cho mutant showing smbp RNA in situ hybridisation. (O,P) At the 26-somite stage, WT somites (O) consist largely of fast muscle (red; antibody EB165) under surface slow muscle (green; BAD5), whereas cho mutants (P) displayed severely decreased fast muscle. Scale bars: A-D,I-L, 75 μm; E-H, 25 μm; M,N, 40 μm; O,P, 25 μm. PHENOTYPE:
|
|
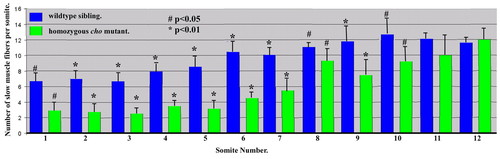
Quantitation of slow muscle fibre numbers at different axial positions at 30 hpf. Student′s t-test P values for comparisons between cho and WT are indicated. Mean±s.d.; n=4. PHENOTYPE:
|
|
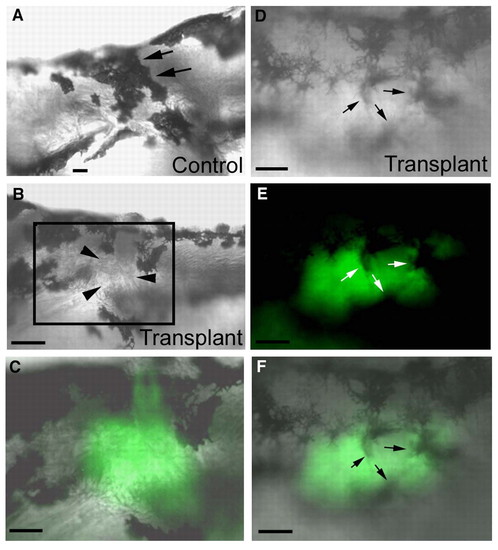
The cho mutant melanophore collar defects are caused by an underlying somite defect. (A-C) WT → cho mutant somite transplant embryo. (A) Right-hand, non-transplanted control side of a cho homozygote showing the melanophore collar at 5 dpf (arrows). Dorsal is to the top, anterior to the right. (B) Left-hand, experimental, transplanted side of the same embryo at the same time as in A. Note the gap in the collar on this side (arrowheads). (C) High magnification of boxed region in B, showing tight correlation of transplanted WT somite from alpha actin-GFP donor (green) with the collar region avoided by melanophores. Anterior is to the left, dorsal to the top. (D-F) The same transplanted WT → cho chimaeric embryo at 24 hours post-transplantation (2 dpf), showing that at early stages melanophores (arrows) traverse the transplanted tissue freely. Lateral views, anterior to the left, dorsal to the top. (D) Collar region on experimental side showing multiple melanophores traversing the transplant. (E) Fluorescent image showing the WT transplanted tissue (green). (F) D and E merged. Scale bars: 130 μm in B; 60 μm in A,C-F. |
|
sdf1a whole-mount mRNA in situ hybridisations in WT and cho mutant. sdf1a is expressed in disk-shaped somite external cells along the horizontal myoseptum (arrowheads) in WT and in the collar region (arrows) in cho mutants. (A) Lateral views at 24, 36 and 55 hpf. (B) Dorsal views at 55 hpf. (C) Transverse cross-sections through the anterior and posterior trunk at 24, 36 and 55 hpf. (D) Confocal images of transverse cross-sections through the anterior and posterior trunk at 27 hpf showing superficial expression of sdf1a mRNA (red) compared with MF20 antibody staining of muscle (green). (E) Higher magnification lateral view of cho anterior trunk at 30 hpf. Scale bars: 25 μm. |
|
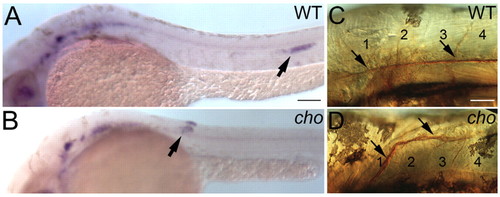
Abnormal primordium migration results in misrouting of the PLL nerve in anterior-most trunk of cho mutant embryos. (A,B) Whole-mount mRNA in situ hybridisation of cxcr4b in 34 hpf WT (A) and cho mutant (B) to show aberrant migration of PLL primordium (arrow). (C,D) This results in misrouting and truncation of PLL nerve (arrow), as seen by zn-12 immunohistochemistry on 56 hpf WT (C) and cho mutant (D) embryos. Somites are numbered. Scale bars: 50 μm in A,B; 20 μm in C,D. |
|
Implantation of Sdf1-soaked beads into WT and cho mutant embryos results in the formation of an ectopic melanophore collar. (A) SDF1-soaked beads (b) were implanted in a dorsal position directly against the skin of a WT embryo at 24 hpf, held in place by a drop of low melting point agarose. Embryos were photographed at 48 hpf to ensure the bead had stayed in place and to record the location of the bead. Agarose holding the bead distorts light transmission, causing the halo evident in this image. (B) Same embryo as in A but at 72 hpf, after bead removal to document the phenotype. The position at which the bead was placed is marked with an arrow. Melanophores have accumulated ectopically, adjacent to the location of the bead implant. (C) Low magnification of same larva as in B. (D,E) Two independent WT embryos, treated similarly, also show ectopic melanophore accumulations adjacent to the location of the SDF1 bead (arrows). Ectopic melanophores form regardless of where in the embryo the bead is positioned. Insert in E is the same animal sectioned in an area containing ectopic melanophores, immunolabelled for MyHc (brown) and demonstrating that bead implantation did not affect muscle differentiation or maintenance. Note that beads are not surgically implanted, but merely placed on the skin; thus, no wound is formed. (F) Implantation of SDF1 beads in homozygous cho mutant embryos (note collar, arrowhead) results in similar accumulation of ectopic melanophores adjacent to site of bead (arrow). Scale bars: 100 μm in A,B; 350 μm in C-F; 25 μm in insert. |
|
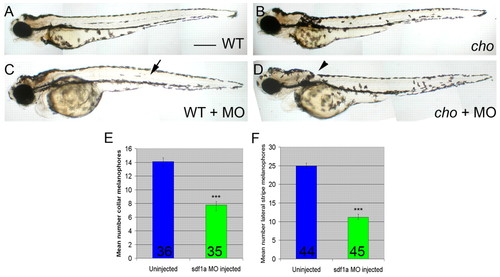
Morpholino-mediated knock-down of sdf1a generates melanophore patterning defects in 55 hpf embryos. (A-D) Fixed cho mutant embryos (B,D) and WT siblings (A,C) with (C,D) or without (A,B) sdf1a morpholino. Note the decreased numbers of melanophores in the WT lateral stripe (arrow) and the cho collar (arrowhead) after morpholino injection. Scale bar: 375 μm. (E,F) Quantitation of these defects. (E) Lateral pathway collar melanophores of cho mutant embryos. (F) Lateral stripe melanophores of WT siblings. Bar graphs show mean±s.e. melanophore counts per embryo; number of embryos is indicated at base of bar. WT and cho mutants were identified by their anterior trunk myotome phenotypes. Reduction of cho mutant collar and WT lateral stripe melanophores were both highly significant; ***, Student's t-test P<0.0001. PHENOTYPE:
|
|
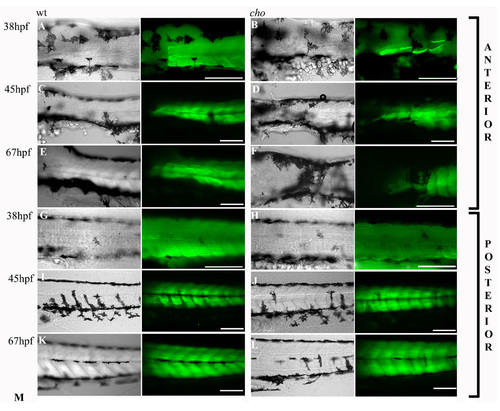
Progressive nature of the muscle fibre defect in cho homozygotes. Slow muscle fibre arrangements and integrity as revealed by labelling with slow MyHc antibody (green). Left frame is a DIC image showing melanophore positions. The right frame combines this DIC image with a fluorescent image revealing F59 antibody labelling in one focal plane. Anterior trunk (A-F) and posterior trunk (G-L) are shown for WT (A,C,E,G,I,K) and cho mutant (B,D,F,H,J,L) embryos at 38 (A,B,G,H), 45 (C,D,I,J) and 67 hpf (E,F,K,L). Scale bars: 125 μm. |

Unillustrated author statements |