- Title
-
Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons
- Authors
- Guo, S., Wilson, S.W., Cooke, S., Chitnis, A.B., Driever, W., and Rosenthal, A.
- Source
- Full text @ Dev. Biol.
|
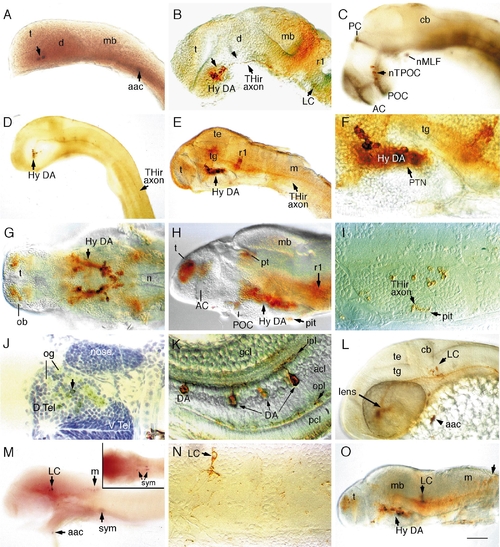
Catecholaminergic neurons in the brain of zebrafish embryos. Whole-mount embryos with rostral to the left (except I, J, and N), labeled with digoxygenin–RNA antisense probes to TH (A) and DβH (M) or antibodies to TH (rest of the images). (A) TH-positive neurons in a 20- to 23-somite embryo. (B) Hypothalamic DA neurons in a prim-12 (28 hpf) embryo are present rostral to the ventral flexure (indicated by an arrowhead) in the ventral diencephalon (the hypothalamus). Locus coeruleus (LC) neurons are present adjacent to rhombomere 1 (r1). (C) DA neurons are distributed along the TPOC (labeled in black with anti-acetylated tubulin antibody) in a prim-12 embryo. (D) Lateral view of a prim-12 embryo showing THir axons extending to the rostral spinal cord. (E–G) Lateral (E–F) and ventral (G) views of THir neurons in 50-h (E and F) and 72-h (G) embryos. (H–I) Lateral view and transverse section of the THir hypothalamus in a 96-h fry. Axonal labeling in the pituitary is indicated in I. (J) Cross section of THir telencephalon in a 96-h fry showing dopaminergic innervation of olfactory glomerular structures. (K) DA amacrine neurons in the retina of a 96-hpf fry. (L) THir LC neurons and arch-associated CA cells (aac) in a prim-15 embryo (30 h). (M) Labeling of a 48-h embryo with DβH RNA probe shows that LC, caudal hindbrain, arch-associated, and sympathetic neurons are noradrenergic. Inset depicts sympathetic neurons in the plane of focus. (N) Transverse section showing LC neuron in a 96-h fry with polarized axonal projection. (O) A 96-hpf fry showing THir neurons in the caudal medulla. Abbreviations: aac, arch-associated CA cells; AC, anterior commissure; acl, amacrine cell layer; cb, cerebellum; d, diencephalon; D.Tel, dorsal telencephalon; V.Tel, ventral telencephalon; gcl, ganglion cell layer; hy, hypothalamus; ipl, inner plexiform layer; LC, locus coeruleus; m, medulla; mb, midbrain; n, notochord; nMLF, nucleus of the medial longitudinal fascicle; nTPOC, nucleus of the tract of postoptic commisure; ob, olfactory bulb; og, olfactory glomerule; opl, outer plexiform layer; PC, posterior commisure; pcl, phororeceptor cell layer; pit, pituitary; POC, postoptic commisure; pt, pretectum; PTN, posterior tubercular nuclei; r1, rhombomere 1; sym, sympathetic neurons; t, telencephalon; te, tectum; tg, tegmentum. Scale bar: 90 μm (A, B, C, G, H, L), 180 μm (D, E, M, O), 45 μm (F, I, J, N), and 30 μm (K). EXPRESSION / LABELING:
|
|
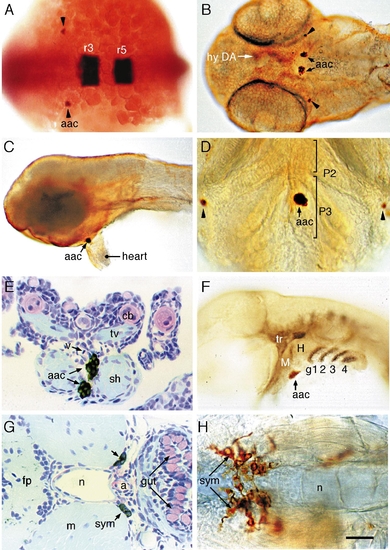
Catecholamine cell groups in the peripheral nervous system of zebrafish embryos. Whole-mount embryos or transverse sections labeled with anti-TH antibody or antisense TH RNA probe. (A) TH-positive cells (indicated with black arrowheads) adjacent to the rostral hindbrain of 22S embryo—dorsal view. Rhombomeres r3 and r5 are labeled by Krox-20. (B–D) Ventral (B and D) and lateral (C) views of THir cells in 50-h (B and C) and 72-h (D) embryos. Arrowheads indicate THir cells posterior to the eyes. Rostral is up in D. (E) Transverse section through the pharyngeal arches of a 96-h fry showing THir cells located between the sternohyoid muscles of arch 3. (F) Prim-25 (36 h) embryo showing THir cells located at the interface between primordial arches 2 and 3, rostral to the gill arches (labeled in black with zn-8 antibody). (G) Putative sympathetic THir cells ventral and lateral to the rostral notochord of a 96-h fry. (H) Sympathetic (sym) THir neurons in a 96-h fry. Abbreviations: a, aorta; aac, arch-associated CA cells; cb, ceratobranchial; fp, floor plate; g1, 2, 3, and 4, gills 1, 2, 3, and 4; H, hyoid; M, mandibular; m, muscle; n, notochord; P2 and P3, pharyngeal arches 2 and 3; sh, sternohyoid; sym, sympathetic neurons; tr, trigeminal ganglia; tv, transverse ventral muscle; v, blood vessel. Scale bar: 80 μm (A), 100 μm (B, C, F), 50 μm (D, H), and 25 μm (E, G). EXPRESSION / LABELING:
|
|
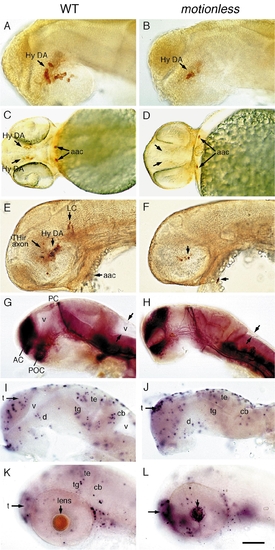
motionless embryos have reduced numbers of hypothalamic DA neurons and exhibit localized patterns of apoptosis. Lateral views (except C and D) of wildtype (left) and mot (right) embryos labeled with (A–F) anti-TH antibody, (G and H) anti-acetylated tubulin, or (I–L) TUNNEL stain. (A and B) 30-h embryos showing reduced hypothalamic DA neurons. (C and D) Ventral view showing a mild cyclopic brain, reduced hypothalamic DA neurons, and normal aac in 2-day-old mot embryos. (E and F) 2-day-old embryos showing few poorly differentiated hypothalamic THir cells and absent LC. (G and H) 30-h embryos showing that the major axonal tracts are grossly intact and the absence of brain ventricles. (I and J) 33-h mot embryos do not display excessive cell death compared to wildtype siblings. (K and L) 52-h embryos showing restricted cell death in the telencephalon and lens of the mot embryos. Abbreviations: aac, arch-associated CA cells; AC, anterior commisure; cb, cerebellum; Hy DA,hypothalamic dopaminergic neurons; LC, locus coeruleus; PC, posterior commisure; POC, postoptic commisure; t, telencephalon; te, tectum; tg, tegmentum; v, ventricle. Scale bar: 55 μm (A, B, I–L) and 110 μm (C, D, E, F, G, H). PHENOTYPE:
|
|
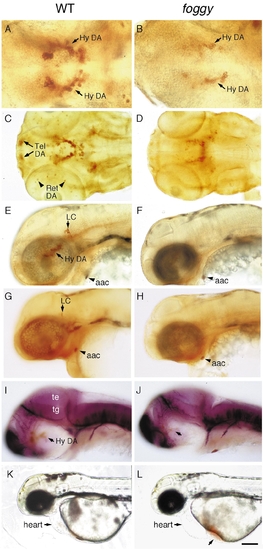
foggy mutant embryos have reduced DA neurons in the telencephalon, retina, and hypothalamus. Wildtype (left) and foggy mutant (right) embryos labeled with antibodies to (A–F) TH, (G and H) DβH, or (I and J) tubulin. (A and B) Ventral views of 2-day-old embryos showing reduced THir and number of hypothalamic DA neurons. (C and D) Ventral view of 3-day-old embryos showing the absence of THir telencephalic and retinal DA neurons. (E–H) Lateral views of 2-day-old embryos showing the absence of LC. (I and J) Lateral views of 36-h embryos showing normal axonal tracts. (K and L) 2-day-old live embryos depict accumulation of blood ventral to the heart due to lack of circulation. Abbreviations: aac, arch-associated CA cells; Hy DA, hypothalamic dopaminergic neurons; LC, locus coeruleus; te, tectum; tg, tegmentum; tel, telencephalon; ret, retina. Scale bar: 48 μm (A, B), 96 μm (C–J), and 192 μm (K, L). EXPRESSION / LABELING:
|
|
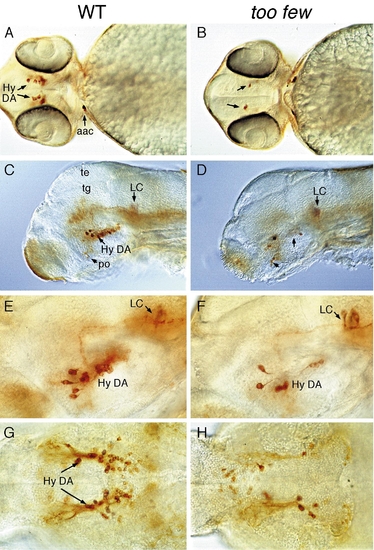
too few mutant embryos have reduced number of hypothalamic neurons. (A, B, and G, H) Ventral and (C–F) lateral views of wildtype (left) and too few mutant (right) embryos labeled with anti-TH antibody. (A–F) 2-day-old embryos showing reduced number of hypothalamic DA neurons but normal complement of postoptic DA and LC neurons. (G and H) 96-h fry showing that the reduction of hypothalamic DA neurons persist. Abbreviations: Hy DA, hypothalamic DA neurons; po, postoptic region; te, tectum, tg, tegmentum; Scale bar: 150 μm (A, B), 120 μm (C, D), and 60 μm (E–H). |
|
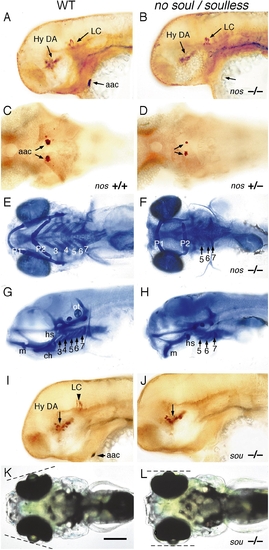
no soul and soulless mutant embryos lack arch-associated CA cells. (A, B, and G–J) Lateral, (C–F) ventral, and (K and L) dorsal views of (A, C, E, G, I, and K) wildtype, (B, D, F, and H) no soul, and (J and L) soulless embryos. Embryos labeled with (A–D, I, and J) anti-TH antibody or (E–H) Alcian blue. (A and B) 2-day-old no soul embryos (B) showing the lack of aac but normal hypothalamic DA and LC neurons. (C and D) 2-day-old heterozygous no soul embryos (D) showing reduced size of aac clusters. (E–H) Cartilage derivatives of arch 2 are reduced (a small hyosymplectic may be present) and arches 3 and 4 are absent in a 5-dpf no soul fry. E and F are ventral and G and H are lateral views. (H) Elements of arch 1 are present, but displaced more ventrally (H). (I and J) 2-day-old soulless mutant embryos (J) showing absence of aac and LC neurons. (K and L) 96-h live soulless fry (J) showing abnormal positioning of the eyes. Abbreviations: aac, arch-associated CA cells; Hy DA, hypothalamic DA neurons; LC, locus coeruleus; P1–7, pharyngeal arches 1–7. Scale bar: 125 μm (A–D, I, J) and 200 μm (E, H, K, L). EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Developmental Biology, 208, Guo, S., Wilson, S.W., Cooke, S., Chitnis, A.B., Driever, W., and Rosenthal, A., Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons, 473-487, Copyright (1999) with permission from Elsevier. Full text @ Dev. Biol.