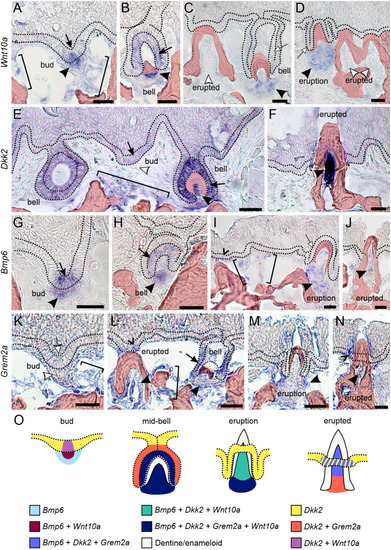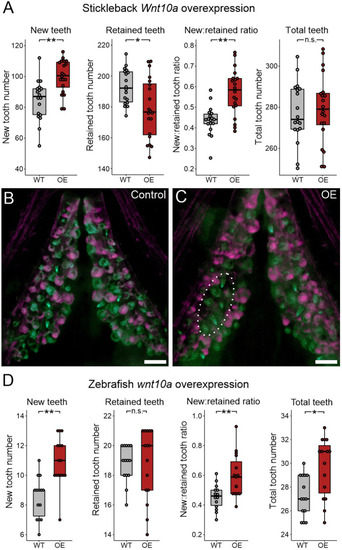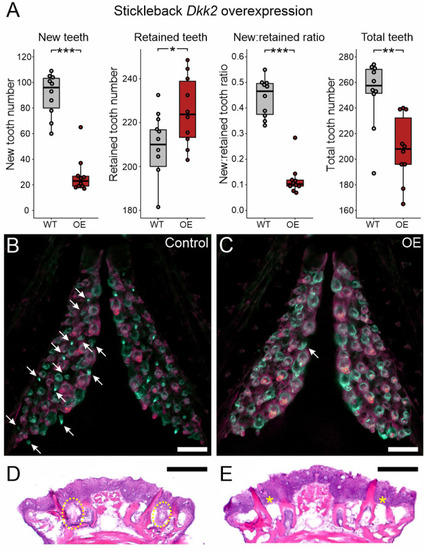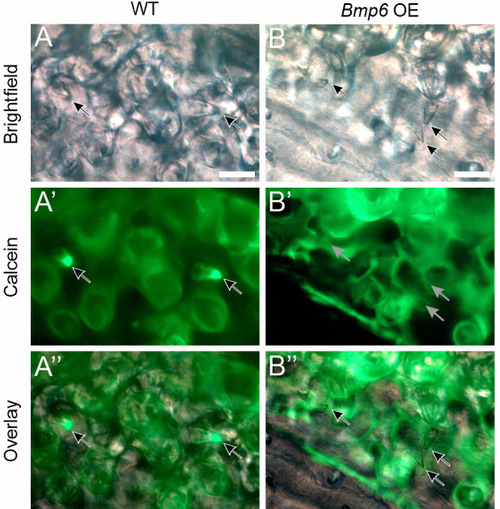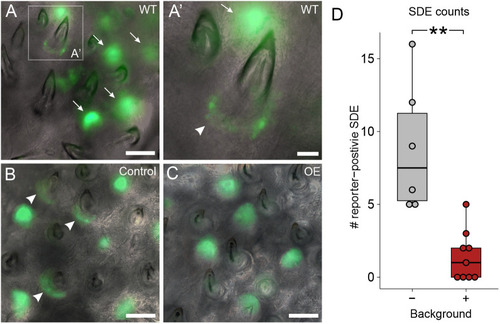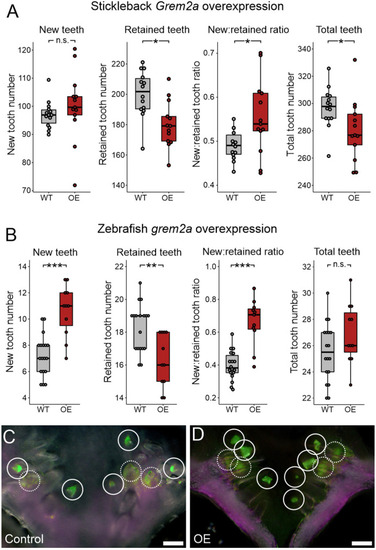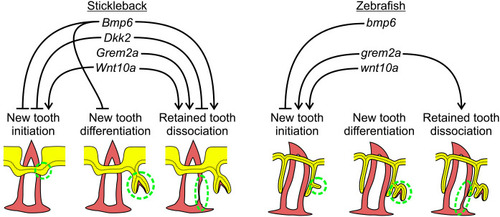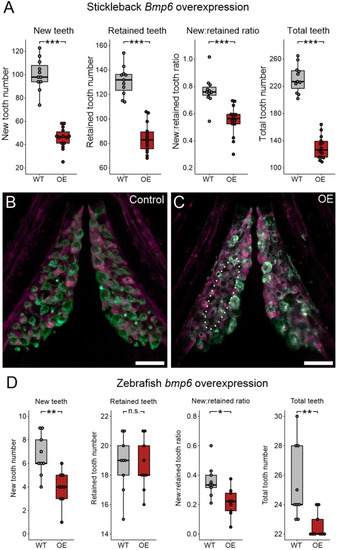
Bmp6 overexpression limits new tooth development in stickleback and zebrafish. All P-values are derived from Wilcoxon Rank-Sum tests, adjusted for multiple hypothesis testing. (A) In sticklebacks, significant decreases in all four variables were detected: the number of new teeth (P=3.7e−5), the number of retained teeth (P=3.7e−5), the new:retained ratio (P=4.4e−5) and total teeth (P=3.7e−5), (n=11 control, 14 OE fish). (B,C) Overlay images of stickleback ventral tooth plates showing Alizarin Red and calcein signal in control (B) and OE (C) fish. Note that regions usually populated with ankylosed teeth are devoid of any such structure (dotted oval in D). Right side is unlabeled. (D) In zebrafish, significant decreases in the number of new teeth (P=0.0084), the new:retained ratio (P=0.018) and total teeth (P=0.0084) were detected, but retained tooth number (P=0.79) did not significantly change (n=9 control, 9 OE fish). Boxes represent the 25th-75th percentiles, the median is shown as a black bar, and whiskers represent those data within 1.5× the interquartile range above and below the 75th and 25th percentiles, respectively. *P<0.05, **P<0.01, ***P<0.001. n.s., not significant. Scale bars: 100 μm.
|