- Title
-
Dimensionality reduction reveals separate translation and rotation populations in the zebrafish hindbrain
- Authors
- Feierstein, C.E., de Goeij, M.H.M., Ostrovsky, A.D., Laborde, A., Portugues, R., Orger, M.B., Machens, C.K.
- Source
- Full text @ Curr. Biol.
|
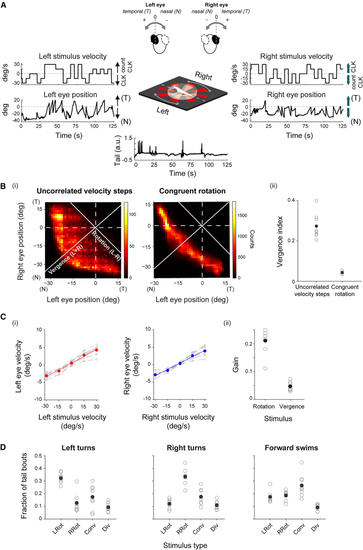
Split motion stimuli are used to decouple eye movements (A) Schematic of visual stimulation (center). Eye rotations are elicited by rotating radial patterns, split into two hemifields, and projected onto a screen placed below the fish (see (B) (i) Left and right eye positions are decorrelated when using a split stimulus (left, uncorrelated velocity steps). In comparison, they are highly correlated when using a whole-field rotating grating (right, congruent rotation). (ii) Vergence index for fish presented with split, incongruent motion stimuli (8 fish) and congruent rotation stimuli (5 fish). Filled circles, mean across fish. Open circles, individual fish. (C) Eye velocity is modulated by stimulus velocity (see also (D) Swimming bout type is modulated by stimulus category (see also See also |
|
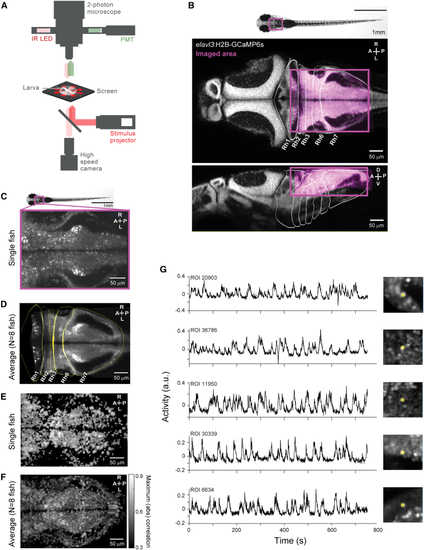
Activity related to behavior is widespread in the zebrafish hindbrain (A) Schematic of experimental setup (see (B) Representative plane highlighting the imaged region (magenta) overlaid on the corresponding plane of the Z-brain atlas. (C) Example plane of the imaged area in a representative fish. (D) Example plane of the average image stack across eight fish (see (E) Maximum absolute correlation value with any of the expanded set of behavioral regressors (see (F) Average maximum absolute correlation maps across eight fish, after registration to an internal template (see (G) Example traces of ROIs used in the analysis. See also |
|
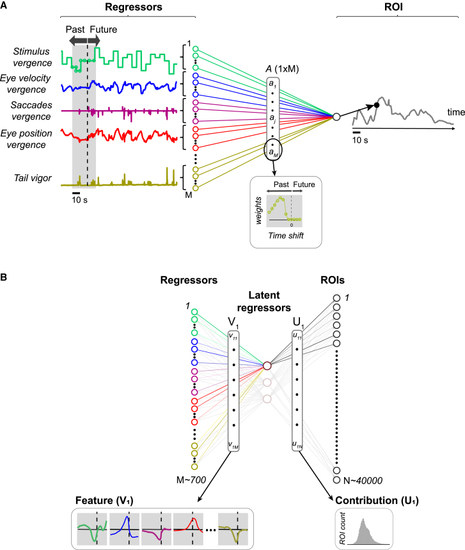
Regression models (A) Multiple linear regression model for a single ROI. For each original variable (only a subset shown here, see (B) Reduced-rank regression. Each time-shifted regressor is first mapped onto a set of latent regressors. The pattern of weights (V1) that determines the mapping for one latent regressor is called a See also |
|
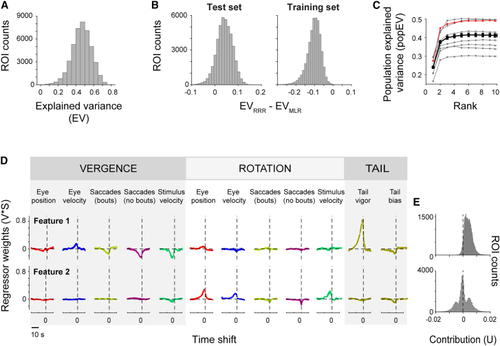
Activity is explained by a small number of features that separate vergence and rotation variables (A) Explained variance (EV; cross-validated) for the ROIs of an example fish using RRR. (B) The cross-validated explained variance of the best RRR model (EVRRR) was generally higher than the explained variance of the best MLR model (EVMLR) (left). Right shows performance on training data, i.e., without cross-validation. Same fish as in (A). (C) Population-explained variance (popEV, cross-validated; see (D) The first two features for the example fish. Feature traces are scaled by their overall importance (V∗S; see (E) Contribution of the latent regressors associated with features 1 (vergence) and 2 (rotation) to ROI activity for the same example fish (43,622 ROIs). See also |
|
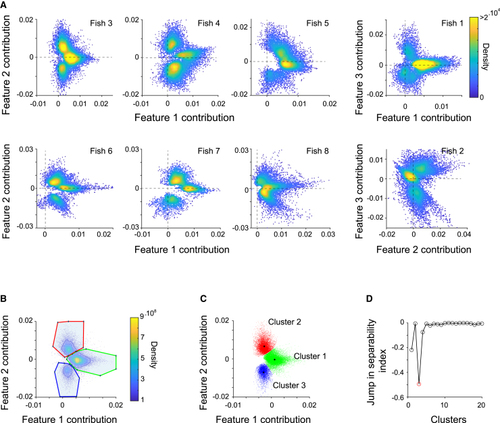
Equivalent ROI clusters are found across all imaged fish (A) Contributions (U) to ROI activity of latent regressors associated with vergence and rotation features group into three clusters. Each dot corresponds to an ROI, each panel to a different fish. Only ROIs with EV > 0.4 are included. (B) Manual assignment of ROIs to three clusters in feature contribution (U1-U2; see (C) ROIs for the example fish are color-coded according to their cluster assignment (ROIs are subsampled here for better visualization). Black asterisks mark cluster centroids. (D) Using ClusterDv |
|
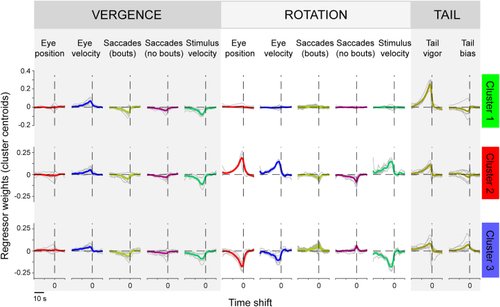
Clusters relate to vergence and left/right rotational motion The weighted contribution of features 1–3 is shown for each cluster’s centroid. Each row corresponds to a cluster, and variables are grouped into vergence, rotation, and tail variables (compare to See also |
|
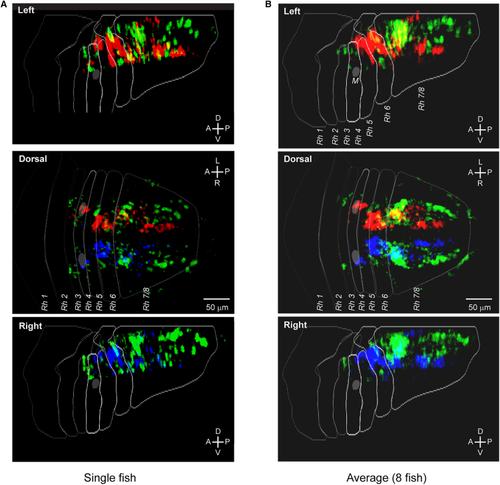
Anatomical distribution of ROIs belonging to the vergence and rotation clusters (A) Spatial distribution of the ROIs assigned to the three clusters in the example fish (32,228 ROIs with EV > 0.4). (B) Average distribution of the ROIs assigned to the three clusters (n = 8 fish). Individual maps (see See also |