- Title
-
Surface Electrical Impedance Myography Detects Skeletal Muscle Atrophy in Aged Wildtype Zebrafish and Aged gpr27 Knockout Zebrafish
- Authors
- Rutkove, S.B., Chen, Z.Z., Pandeya, S., Callegari, S., Mourey, T., Nagy, J.A., Nath, A.K.
- Source
- Full text @ Biomedicines
|
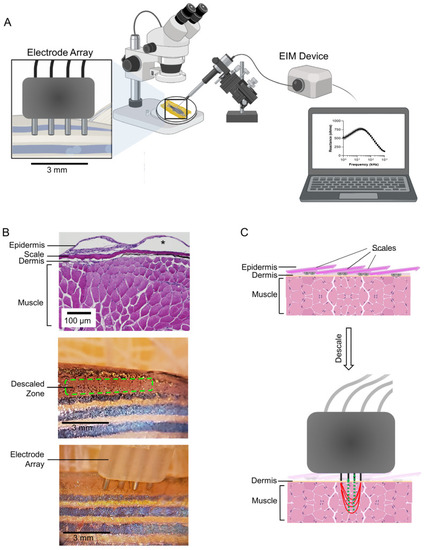
Surface electrical impedance myography in zebrafish skeletal muscle: Experimental set-up. ( |
|
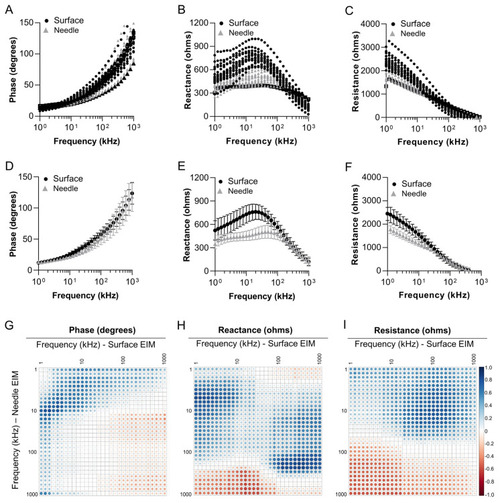
Relationships between surface EIM and needle EIM in adult zebrafish skeletal muscle. Multifrequency (1 kHz–1 MHz) graphs for EIM parameters of ( |
|
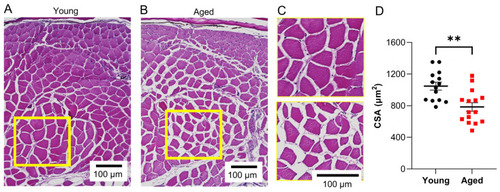
Age-related atrophy of skeletal muscle fibers in wildtype zebrafish. Representative H&E-stained images depicting epaxial caudal musculature in ( |
|
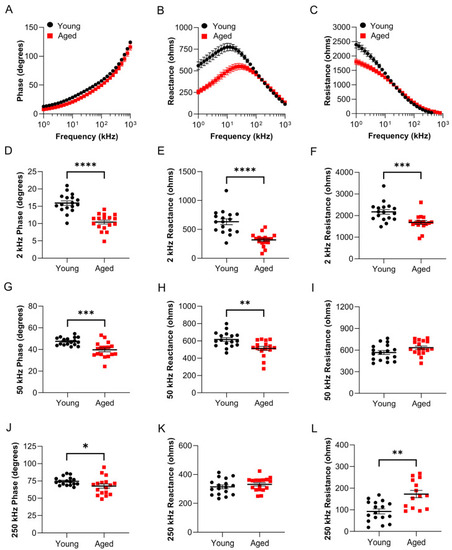
Surface electrical impedance myography detects age-related muscle changes in wildtype zebrafish. sEIM parameters (phase, reactance, and resistance) were assessed at a range of frequencies (1 kHz–1 MHz) in the epaxial caudal muscles of young (8 months, ~20% lifespan; |
|
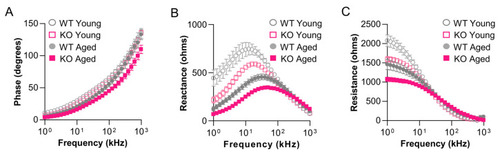
Aged |
|
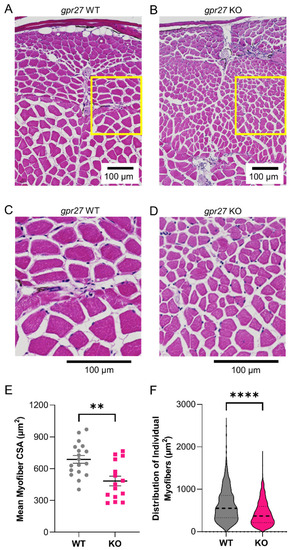
Genetic deletion of |
|
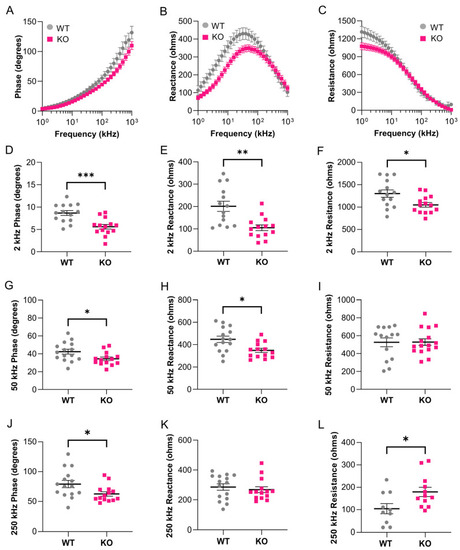
Surface electrical impedance myography detects skeletal muscle defects in young |
|
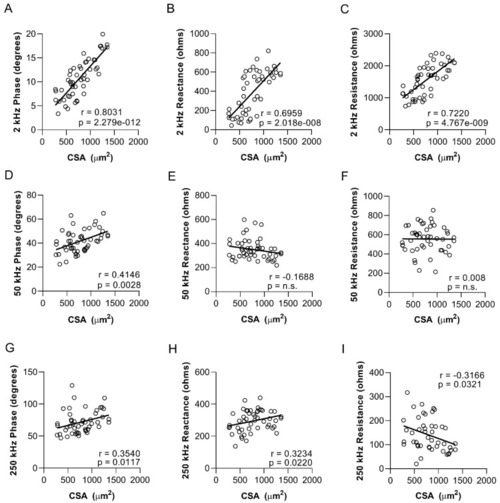
Myofiber size in zebrafish skeletal muscle strongly correlates with low-frequency phase, reactance, and resistance values. Correlation between cross-sectional myofiber area in the epaxial caudal musculature and sEIM values: ( |
|
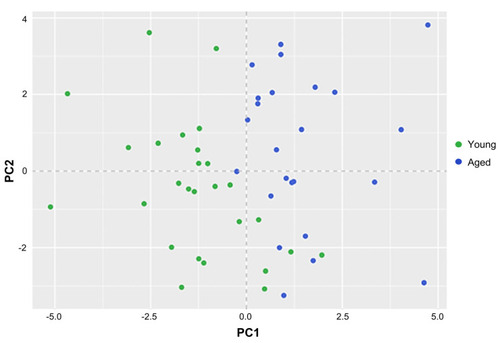
Principal component analysis of EIM measurements in young and aged zebrafish. Nine EIM parameters were used in this analysis (phase: 2, 50, and 250 kHz; reactance: 2, 50, and 250 kHz; and resistance: 2, 50, and 250 kHz). Data from 51 animals was used ( |