- Title
-
Macrophages undergo a behavioural switch during wound healing in zebrafish
- Authors
- Sipka, T., Park, S.A., Ozbilgic, R., Balas, L., Durand, T., Mikula, K., Lutfalla, G., Nguyen-Chi, M.
- Source
- Full text @ Free Radic. Biol. Med.
|
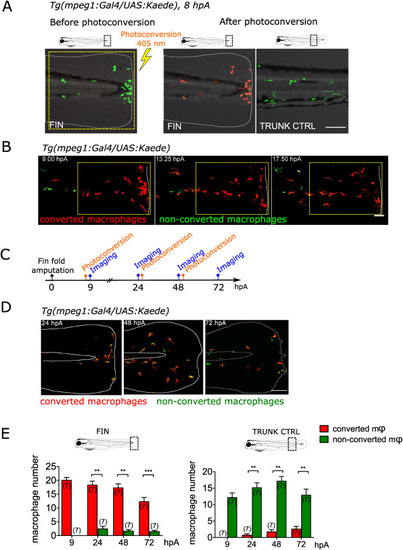
Fig. 1. Same population of macrophages is present at the wound during healing and repair (A) The caudal fin fold of Tg(mpeg1:Gal4/UAS:Kaede) larvae was amputated at 3 dpf. Recruited Kaede+ macrophages (green) were photo-converted to red fluorescence using UV at using 403 nm laser illumination in the wound region at 8 hpA. Representative confocal images of the fin fold before the photoconversion (left) and after the photoconversion (middle). On the right, control image of the trunk region taken after the photoconversion, outside of the wound area showing non-photoconverted macrophages (green). Representative merged images of the Kaede fluorescence in the green and red channels and bright field images. Yellow dashed line indicates the photoconverted area. The white lines outline the fin fold. Scale bar: 100 μm. (B) Representative frames (Maximum projection) of the time lapse imaging of photoconverted area, spanning from 9 to 19 hpA, with a time step of 10 min. White dashed line indicate the wound margin. Scale bar: 50 μm. (C) Schedule of the photoconversion pulse-chase experiment. Fin folds of Tg(mpeg1:Gal4/UAS:Kaede) larvae were amputated at 3 dpf. Photoconversions of Kaede+ macrophages were performed at 8, 25 and 49 hpA. Images were acquired at 9, 24, 48 and at 72 hpA. (D) Representative maximum projections of fin folds at 24, 48 and 72 hpA, taken before the new photoconversion; Merged images of the Kaede fluorescence (green and red channels). The white lines outline the fin fold and the notochord. Scale bar: 100 μm. (E) Quantification of photoconverted (red) and non-photoconverted (green) macrophages (Φ) at the wound (left) and in control trunk region (right), in indicated time point. Two independent experiments, number of larvae is indicated in the figure, Mann-Whitney test, two-tailed, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
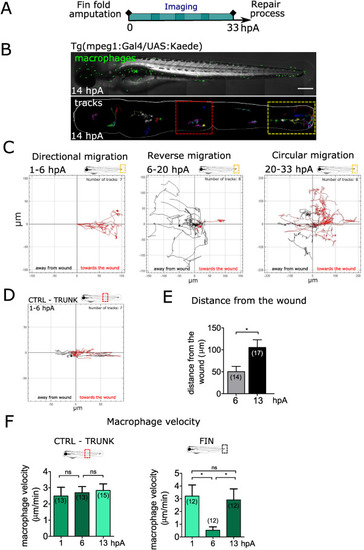
Fig. 2. Macrophages undergo a stereotypical migratory behaviour at the wound (A) Schedule of the experiments. Tg(mpeg1:gal4/UAS:Kaede) larvae with mosaic expression of Kaede protein in macrophages were amputated at 3 dpf and imaged using high resolution Spinning Disk microscopy for one of consecutive periods lasting between 5 and 15 h, that together spanned 72 h of regeneration spanning 3 days of regeneration. (B) Up: representative frame from 5.5 to 14 hpA time-lapse video of mosaically labelled macrophage population in the whole larva (14 hpA). Scale bar: 250 μm. Down: Representative tracks of macrophages representing the paths of individual macrophages from 5.5 to 14 hpA. (C) Macrophage migration directionality, in the wound region, during defined periods showing different migration modes: directional (period 1–6 hpA), reverse (period 6–20 hpA) and circular migration (period 20–33). The tracks with the directionality toward the wound are coloured in red, while the tracks with the directionality away from the wound are black. Number of macrophage tracks and the time period covered by graph are indicated above each graph. (D) Macrophage migration directionality in the unaffected trunk region (CTRL). The tracks with the directionality toward the wound are coloured in red, while the tracks with the directionality away from the wound are black. Number of macrophage tracks is indicated above the graph. (E) Quantification of wound distance value corresponding to the shortest distance measured from the wound margin to the center of each individual macrophage at 6 and 13 hpA. Number of macrophages is indicated in parenthesis. (mean±standard error of the min (sem)) Representative experiment of five independent experiments, two-tailed paired t-test, *p < 0.05. (F) Quantification of individual macrophage velocity at the control (CTRL) trunk region (left) and at the wound at 1, 6 and 13 hpA. Number of analysed macrophages is indicated in the graph. (mean±sem) Representative experiment of three independent experiments, two-tailed paired t-test, *p < 0.05, ns – not significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
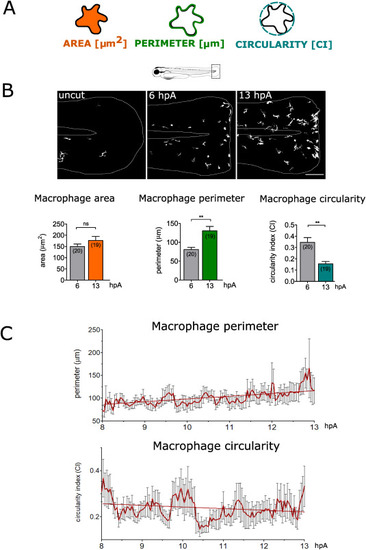
Fig. 3. Macrophages change their morphology at the wound (A) Representation of 2-D morphometric descriptors used for macrophage shape analysis: area, perimeter and circularity. (B) Up: Representative images of non-amputated and amputated fin folds of Tg(mpeg1:Gal4/UAS:Kaede) larvae at 6 and 13 hpA; confocal maximum projections of keade fluorescence (white) in macrophages. White lines outline the fin fold. Down: Quantification of area, perimeter and circularity for individual macrophages at 6 and 13 hpA. Number of analysed macrophages is indicated in parenthesis. (mean±sem) Representative experiment of five independent experiments, two-tailed paired t-test, **p < 0.01, ns – not significant. (C) Quantification of perimeter (upper graph) and circularity value (lower graph) for individual macrophages present at the wound, during a time lapse sequence from 8 hpA to 13 hpA every 2.5 min (mean±sem). |
|
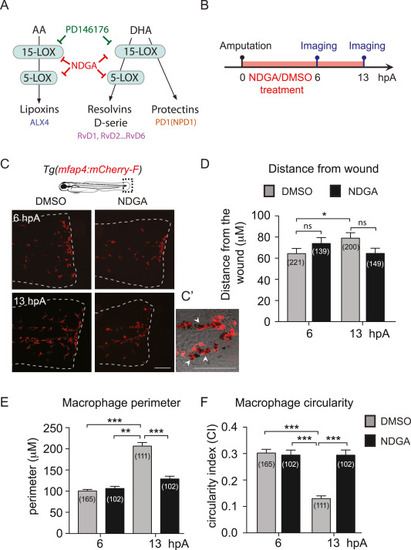
Fig. 4. Inhibition of Lipid metabolism via NDGA alters macrophage behavioural switch (A) The biosynthetic pathways of SPM leading to lipoxins, D-series resolvins and Protectins. Lipoxins (LXA4) are derived from arachidonic acid (AA) through the action of 15-Lipoxygenase (15-LOX) and Lipoxygenase 5 (5-LOX). D-series resolvins (RvD1 … 6) and protectins (PD1) are derived from docosahexaenoic acid (DHA) by the action 15-LOX and 5-LOX and from 15-LOX only, respectively. (B) Schedule of the experiment. The fin folds of Tg(mfap4:mcherry-F) larvae were amputated at 3 dpf and immediately treated with DMSO or NDGA 50 μM. Larvae were imaged in live at 6 and 13 hpA. (C) Representative images of the fin folds from amputated Tg(mfap4:mcherry-F) larvae at 13 hpA after DMSO or NDGA 50 μM treatment. Images are maximum projections from confocal microscopy showing the fluorescence of mCherry-F (macrophages) in the fin folds. White lines outline the fin fold. Scale bar: 100 μm. (C′) Zoomed region of the wound at 13 hpA after NDGA 50 μM treatment. Maximum projection from confocal microscopy z-stack showing the fluorescence of mCherry-F (macrophages) overlaid on a brightfield image. Arrow heads show macrophages around pigments. Scale bar: 100 μm (D) Quantification of macrophage distance from the wound (values in μm) in DMSO or NDGA -treated larvae at 6 and 13 hpA. Mean±sem, three independent experiments, the number of analysed macrophages in parenthesis, kruskal-Wallis test with Dunn's post-test, ***p < 0.001, ns – not significant. (E) Quantification of macrophage perimeter in DMSO or NDGA -treated larvae at 6 and 13 hpA. Mean±sem, three independent experiments, the number of analysed macrophages is indicated in parenthesis, kruskal-Wallis test with Dunn's post-test, **p < 0.01, ***p < 0.001. (F) Quantification of macrophage circularity in DMSO or NDGA -treated larvae at 6 and 13 hpA. Mean±sem, three independent experiments, the number of analysed macrophages is indicated in parenthesis, kruskal-Wallis test with Dunn's post-test, ***p < 0.001. |
|
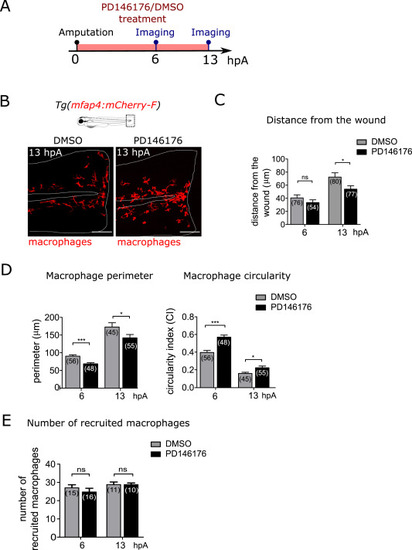
Fig. 5. Lipoxygenase 15 activity is required for macrophage behavioural switch (A) Schedule of the experiment. The fin folds of Tg(mfap4:mcherry-F) larvae were amputated at 3 dpf and immediately treated with DMSO or PD146176. Larvae were imaged in live at 6 and 13 hpA. (B) Representative images of the fin folds from amputated Tg(mfap4:mcherry-F) larvae at 13 hpA after DMSO or PD146176 treatment; Maximum projections from confocal microscopy showing mCherry-F macrophages in the fin folds. White lines outline the fin fold and the notochord. Scale bar: 100 μm. (C) Quantification of macrophage wound distance in DMSO- and PD146176-treated larvae at 6 and 13 hpA. Mean±sem, two independent experiments, the number of analysed macrophages from 4 larvae is indicated in parenthesis, two-tailed Mann-Whitney test, *p < 0.05, ns – not significant (D) Quantification of macrophage perimeter and circularity in DMSO- and PD146176-treated larvae at 6 and 13 hpA. Mean±sem, two independent experiments, number of analysed macrophages from 4 larvae is indicated in parenthesis, two-tailed Mann-Whitney test, *p < 0.05, ***p < 0.001. (E) Quantification of the number of recruited macrophages at 6 and 13 hpA, in DMSO- and PD146176-treated larvae. Mean±sem, two independent experiments, number of larvae is indicated in parenthesis. Two-tailed Mann-Whitney test, ns – not significant. |
|
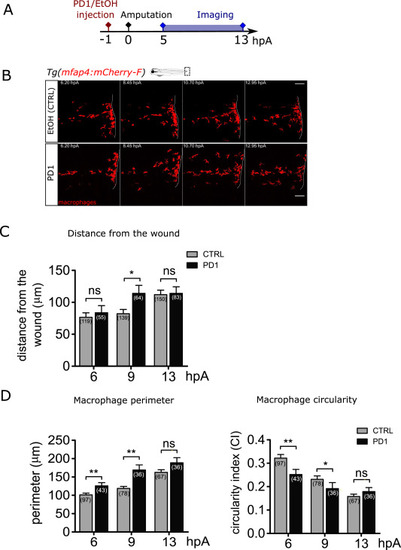
Fig. 6. Synthetic SPM protectin D1 accelerates the macrophage behavioural switch at the wound. (A) Schedule of the experiment. Protectin D1 (PD1) or same volume of ethanol were injected in 3 dpf Tg(mfap4:mCherry-F) larvae, 1 h before the fin fold amputation, and imaged from 5 to 13 hpA. (B) Representative frames of time-lapse imaging of Tg(mfap4:mCherry-F) larvae injected with ethanol as a control (up) or with PD1 (down). White lines indicate wound margin. Scale bars: 100 μm. (C) Quantification of macrophage distance from the wound margin in ethanol - (CTRL) and PD1-injected larvae at 6, 9, and 13 hpA. Number of macrophages is indicated in parenthesis. Mean±sem, from two independent experiments, two-tailed Mann Whitney test, **p < 0.01, ns – not significant. (D) Quantification of macrophage perimeter and circularity in ethanol - (CTRL) and PD1-injected larvae at 6, 9, 10, 11, 12 and 13 hpA. Number of analysed macrophages is indicated in parenthesis; two independent experiments, Mann-Whitney two-tailed test, *p < 0.05, **p < 0.01 and ***p < 0.01. |
|
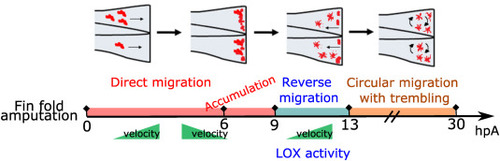
Fig. 7. Diagram of macrophage behavioural switch during wound healing Macrophage behavioural changes during wound healing highlighting 3 different migration modes, velocity changes and shape changes at indicated time points after amputation. First, macrophages undergo a directional migration, with high velocity, followed with accumulation of macrophages at the wound, having low velocity and amoeboid shape. Second macrophages undergo a reverse migration, with the opposite directionality compared to the first phase, occurring between 9 and 13 hpA. This phase also includes increase of velocity and shape changes towards branched, elongated morphology and it is regulated by LOX activity. The third phase is circular migration using mesenchymal mode, with constant high velocity and branched, elongated shape, lasting till the end of wound repair. |
Reprinted from Free radical biology & medicine, 192, Sipka, T., Park, S.A., Ozbilgic, R., Balas, L., Durand, T., Mikula, K., Lutfalla, G., Nguyen-Chi, M., Macrophages undergo a behavioural switch during wound healing in zebrafish, 200-212, Copyright (2022) with permission from Elsevier. Full text @ Free Radic. Biol. Med.