- Title
-
Spontaneously recycling synaptic vesicles constitute readily releasable vesicles in intact neuromuscular synapses
- Authors
- Egashira, Y., Kumade, A., Ojida, A., Ono, F.
- Source
- Full text @ J. Neurosci.
|
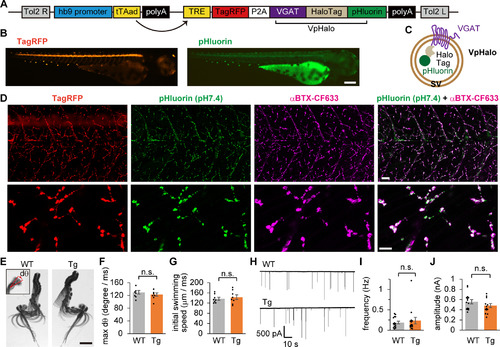
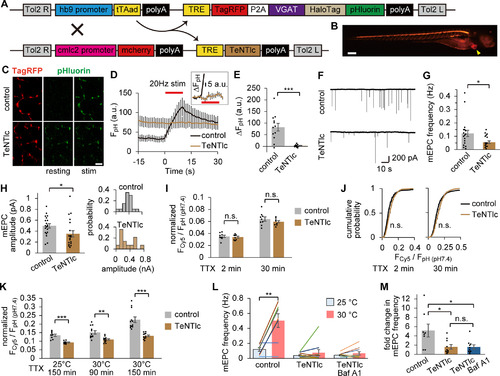
Tg zebrafish expressing two independent indicators of SV recycling in motoneurons. |
|
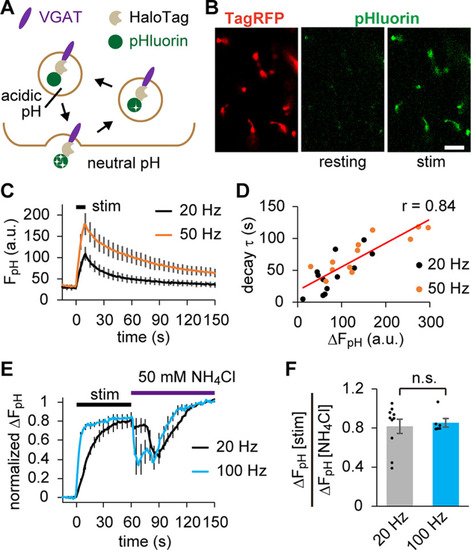
pHluorin live imaging indicated that SVs carrying VpHalo recycle normally in response to APs. |
|
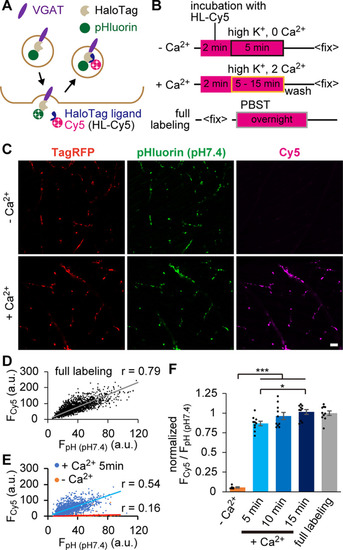
HaloTag-based SV labeling enabled quantifying the fraction of recycled SVs. |
|
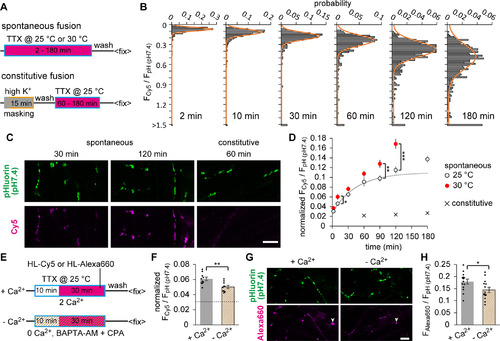
HaloTag labeling of spontaneously recycled SVs suggested two distinct populations of spontaneous fusion. |
|
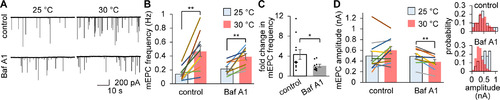
The temperature-dependent increase in mEPC events was greatly impaired by the inhibition of transmitter refilling. |
|
TeNT impaired the late-onset component of spontaneous SV labeling. |
|
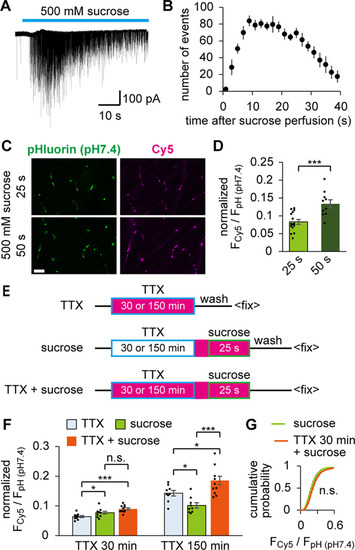
Spontaneously recycling SVs were included in the total recycling pool. |
|
Spontaneously recycled SVs overlapped with RRP mobilized by hypertonic stimulation. |
|
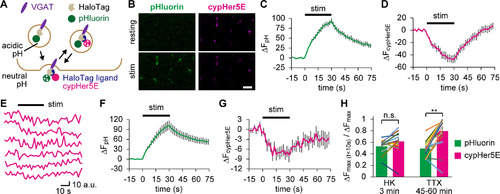
Spontaneously recycled SVs behaved like RRP vesicles in subsequent APs. |
|
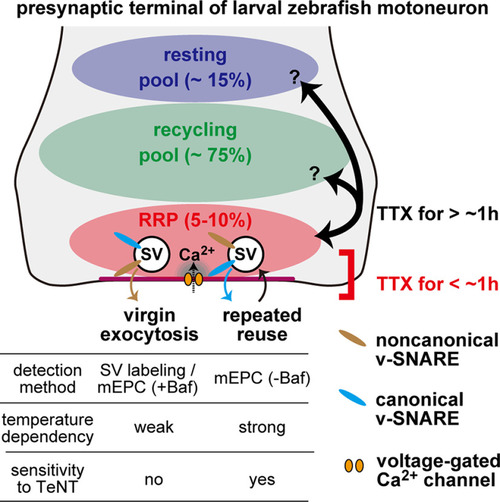
Summary diagram of spontaneous SV fusion at larval zebrafish neuromuscular synapses. Three pools of SVs are depicted: resting pool, recycling pool, and RRP. In the early phase of TTX treatment (<1 h), spontaneous SV fusion is mobilized from the RRP with two distinct modes: virgin exocytosis with a slow time course (τ = 45 min at 25°C) and repeated reuse of the same SVs at a higher rate. The differences between the two modes of fusion are highlighted in the table at bottom. SVs equipped with both canonical and noncanonical v-SNAREs are involved in the two modes, although their dependency on the noncanonical v-SNARE is not identical. They may also be different in their coupling to the voltage-gated Ca2+ channels. SVs in RRP are intermixed with those in other pools after a prolonged (>1 h) TTX treatment. |