- Title
-
Lamb1a regulates atrial growth by limiting second heart field addition during zebrafish heart development
- Authors
- Derrick, C.J., Pollitt, E.J.G., Sevilla Uruchurtu, A.S., Hussein, F., Grierson, A.J., Noël, E.S.
- Source
- Full text @ Development
|
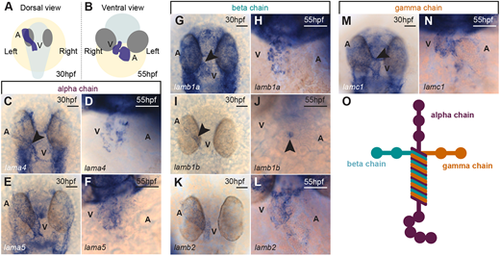
Dynamic expression of laminin subunit genes during heart morphogenesis. (A,B) Schematic of the position of the heart (blue) in a 30 hpf zebrafish embryo (A; dorsal view) and a 55 hpf zebrafish embryo (B; ventral view). The embryo body is shaded pale grey, eyes are shaded dark grey and the yolk is shaded in yellow. (C-F) mRNA in situ hybridisation expression analysis of laminin alpha chain subunits lama4 (C,D) and lama5 (E,F) in the heart. (G-L) mRNA in situ hybridisation expression analysis of laminin beta subunit chains lamb1a (G,H), lamb1b (I,J) and lamb2 (K,L) in the heart. (M,N) mRNA in situ hybridisation expression analysis of gamma subunit lamc1 in the heart. Arrowheads indicate the position of the heart and the anterior is to the top in all images. (O) Schematic of the heterotrimeric structure of laminin. Scale bars: 50 μm. A, atrium; V, ventricle. |
|
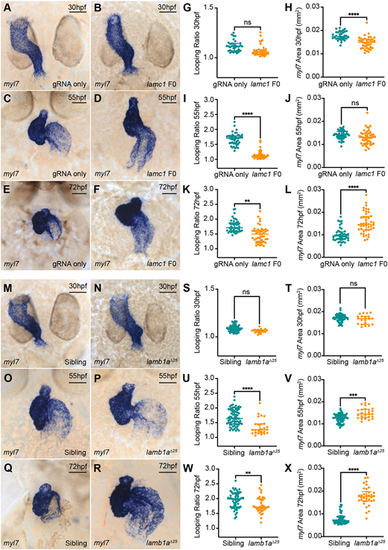
View largeDownload slide Laminins perform multiple roles during zebrafish heart morphogenesis. (A-F) mRNA in situ hybridisation analysis of myl7 expression in control embryos injected with lamc1-targeting gRNAs only (A,C,E) or with lamc1-targeting gRNAs together with Cas9 protein (lamc1 F0; B,D,F) at 30 hpf (A,B), 55 hpf (C,D) and 72 hpf (E,F). (G-L) Quantitative analysis of looping ratio (G,I,K) and myl7 area (H,J,L) in gRNA-injected controls (30 hpf: n=34; 55 hpf: n=44; 72 hpf: n=44) and lamc1 F0 crispants (30 hpf: n=38; 55 hpf: n=47; 72 hpf: n=44). lamc1 crispants exhibit reduced heart looping at 55 hpf and 72 hpf, a reduced area of myl7 expression at 30 hpf and an increased area of myl7 expression at 72 hpf. Data are median±interquartile range, analysed with the Kruskal–Wallis test. (M-R) mRNA in situ hybridisation analysis of myl7 expression in siblings (M,O,Q) and lamb1aΔ25 mutants (N,P,R) at 30 hpf, 55 hpf and 72 hpf. (S-X) Quantitative analysis of looping ratio (S,U,W) and myl7 area (T,V,X) in siblings (30 hpf: n=65; 55 hpf: n=70; 72 hpf: n=56) and lamb1aΔ25 mutants (30 hpf: n=20; 55 hpf: n=25; 72 hpf: n=34). lamb1aΔ25 mutants exhibit a mild reduction in heart looping from 55 hpf, and an increased area of myl7 expression at 55 hpf and 72 hpf. Data are median±interquartile range, S-W were analysed with the Mann–Whitney U test, X was analysed with the Kruskal-Wallis test. ****P<0.0001, ***P<0.001, **P<0.01, ns=not significant in all graphs. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
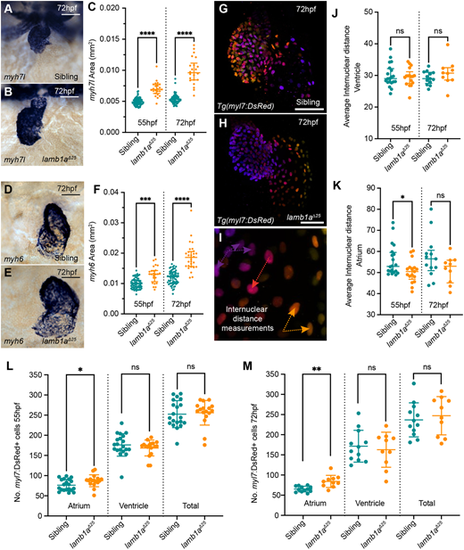
lamb1a mutants have increased atrial cells. (A,B) mRNA in situ hybridisation analysis of myh7l expression in the ventricle of sibling (A) and lamb1aΔ25 mutant embryos (B) at 72 hpf. (C) Quantification of myh7l expression area in sibling (55 hpf: n=72; 72 hpf: n=67) and lamb1aΔ25 mutants (55 hpf: n=23; 72 hpf: n=22). Data are median±interquartile range, analysed with the Kruskal-Wallis test. (D,E) mRNA in situ hybridisation analysis of myh6 expression in the atrium of siblings (D) and lamb1aΔ25 mutants (E) at 72 hpf. (F) Quantification of myh6 expression area in siblings (55 hpf: n=65; 72 hpf: n=64) and lamb1aΔ25 mutants (55 hpf: n=24; 72 hpf: n=29). Data are median±interquartile range, analysed with the Kruskal–Wallis test. (G-I) Depth-coded maximum intensity projections of confocal image z-stacks in Tg(myl7:DsRed) transgenic sibling (G) and lamb1aΔ25 mutants (H) at 72 hpf. Internuclear distance is quantified between nuclei on the same face of the heart that occupy similar z-positions (arrows in I). (J,K) Quantification of average internuclear distance at 55 hpf and 72 hpf in the ventricle (J) and atrium (K) of siblings (55 hpf: n=20; 72 hpf: n=17) and lamb1aΔ25 mutants (55 hpf: n=13; 72 hpf: n=10), demonstrating a mild decrease in the internuclear distance of lamb1aΔ25 mutant atrial cells at 55 hpf (K). Data are median±interquartile range, analysed with Brown–Forsythe and Welch ANOVAs with multiple comparisons. (L,M) Quantification of DsRed+ cells in the myocardium of Tg(myl7:DsRed) transgenic siblings (55 hpf: n=20; 72 hpf: n=17) and lamb1aΔ25 mutants (55 hpf: n=13; 72 hpf: n=10) at 55 hpf (L) and 72 hpf (M). lamb1aΔ25 mutants have a significant increase in atrial cell number at both stages. Data are mean±s.d. Chamber-specific analyses performed with the unpaired t-test with Welch's correction. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant in all graphs. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
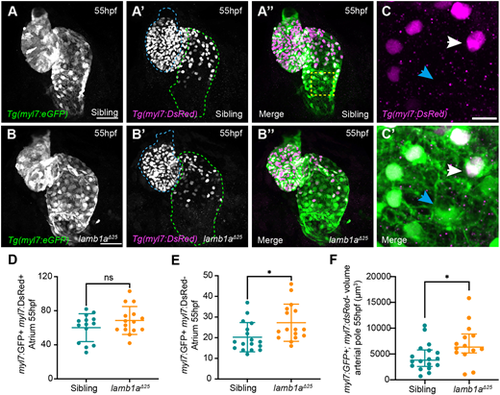
Lamb1a limits SHF addition to the venous pole. (A-B″) Maximum intensity projections of confocal image z-stacks in Tg(myl7:eGFP);Tg(myl7:DsRed) double-transgenic sibling (A-A″) and lamb1aΔ25 mutant embryos (B-B″) at 55 hpf. GFP+;DsRed−SHF cells are visible at the venous (green dotted line) and arterial (blue dotted line) poles of the heart. (C,C′) Higher magnification of the dashed-yellow-boxed area in A″. Double GFP+;DsRed+ cells represent ‘older’ cardiomyocytes (white arrow), whereas GFP+;DsRed− cells represent newly added SHF cells (blue arrowheads). (D,E) Quantification of double GFP+;DsRed+ cardiomyocytes (D) and GFP+;DsRed− SHF cells (E) in the atrium of siblings (n=17) and lamb1aΔ25 mutants (n=16) at 55 hpf reveals an increase in newly added SHF cells in lamb1aΔ25 mutants compared with siblings. Data are mean±s.d., analysed with the Kolmogorov–Smirnov test. (F) Quantification of GFP+;DsRed− myocardial volume in the distal arterial pole in sibling (n=18) and lamb1aΔ25 mutant embryos (n=14) at 55 hpf reveals an increase in SHF myocardium in lamb1aΔ25 mutants compared to controls. Data are median±interquartile range, analysed with Welch's t-test; *=P<0.05, ns=not significant in all graphs. Scale bars: 10 μm in C; 50 μm in A,B. |
|
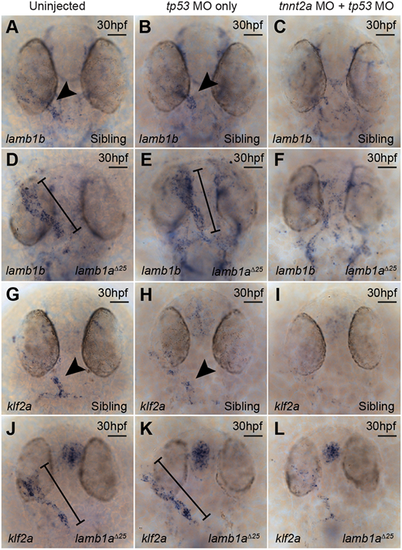
lamb1a mutants exhibit aberrant turbulent flow sensing. (A-F) mRNA in situ hybridisation analysis of lamb1b expression at 30 hpf in sibling (A-C) and lamb1aΔ25 mutant embryos (D-F), either uninjected (A,D), injected with a tp53 MO (B,E) or injected with a tp53 MO+tnnt2a MO (C,F). lamb1b is expressed predominantly in the ventricle/arterial pole of the heart tube endocardium in sibling uninjected (n=39/45) and control tp53 MO-injected embryos (n=43/46) at 30 hpf (arrowheads in A,B), but is lost in embryos injected with tnnt2a MO (C; n=35/40). lamb1b expression is upregulated throughout the endocardium in uninjected (n=23/23) and control tp53 MO-injected lamb1aΔ25 mutants (n=24/28) at 30 hpf (black lines; D,E) compared with sibling controls (arrowheads in A,B). Endocardial lamb1b expression is reduced in lamb1aΔ25 mutants injected with tnnt2a MO (F; n=24/28) compared with control lamb1aΔ25 mutants (D,E). (G-L) mRNA in situ hybridisation analysis of klf2a expression at 30 hpf in siblings (G-I) and lamb1aΔ25 mutants (J-L), either uninjected (G,J), injected with a tp53 MO (H,I) or with a tp53 MO+tnnt2a MO (I,L). klf2a is expressed at low levels throughout the endocardium, with elevated expression at the arterial pole in sibling uninjected (n=42/43) and control tp53 MO-injected (n=37/39) embryos at 30 hpf (arrowheads in G,H), but is lost in embryos injected with tnnt2a MO (I; n=31/49). klf2a expression is upregulated particularly at the venous pole and atrium of lamb1aΔ25 uninjected (n=23/25) and control tp53 MO-injected mutant embryos (n=18/18) at 30 hpf (black lines; J,K) compared with sibling controls (arrowheads in G,H). Endocardial klf2a expression is reduced in lamb1aΔ25 mutants injected with tnnt2a MO (L, n=20/22) compared with control lamb1aΔ25 mutants (J,K). Scale bars: 50 μm |
|
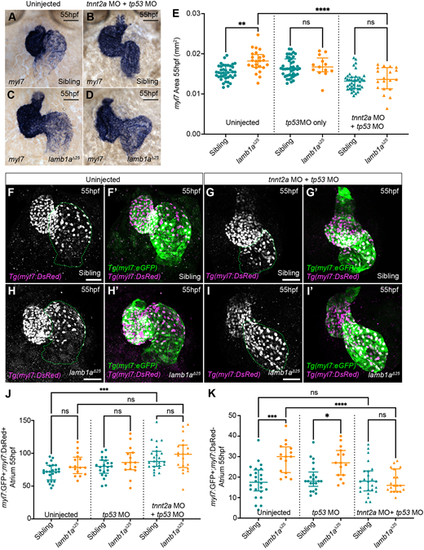
Lamb1a limits excessive, contractility-dependent SHF addition to the venous pole. (A-D) mRNA in situ hybridisation analysis of myl7 expression in sibling (A,B) and lamb1aΔ25 mutant embryos (C,D) either uninjected (A,C) or injected with tp53 MO+tnnt2a MO (B,D). (E) Quantification of myl7 area in uninjected (sibling: n=40; lamb1aΔ25: n=24), tp53 MO-injected control (sibling: n=43; lamb1aΔ25: n=13) and tp53 MO+tnnt2a MO-injected (sibling: n=40; lamb1aΔ25: n=19) embryos at 55 hpf. Data are median±interquartile range, analysed with the Kruskal–Wallis test with multiple comparisons. (F-I′) Maximum intensity projections of confocal image z-stacks in Tg(myl7:eGFP);Tg(myl7:DsRed) double-transgenic sibling (F-G′) and lamb1aΔ25 mutant embryos (H-I′) at 55 hpf, either uninjected (F,F′,H,H′) or injected with tp53 MO+tnnt2a MO (G,G′,I, I′). Green dotted lines indicate the atrium. (J,K) Quantification of double GFP+;DsRed+ atrial cardiomyocytes (J) and GFP+;DsRed− SHF cells (K) at 55 hpf in siblings and lamb1a mutants either uninjected (sibling: n=24; lamb1aΔ25: n=16), injected with tp53 MO (sibling: n=21; lamb1aΔ25: n=15) or injected with tp53 MO+tnnt2a MO (sibling: n=24; lamb1aΔ25: n=20). Blocking heart contractility with the tnnt2a MO rescues excess SHF addition in lamb1a mutants (K). Data are median±interquartile range, analysed with Brown–Forsythe and Welch ANOVAs with multiple comparisons; ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns=not significant in all graphs. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
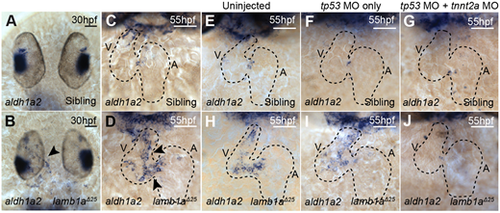
aldh1a2 upregulation in lamb1a mutants is contractility dependent. (A-D) mRNA in situ hybridisation analysis of aldh1a2 expression in sibling and lamb1aΔ25 mutant embryos at 30 hpf (A,B) and 55 hpf (C,D). lamb1aΔ25 mutants exhibit an upregulation of aldh1a2 expression in the endocardium at both stages (arrowheads in B,D; 30 hpf: n=14/20; 55 hpf: n=16/17) compared with siblings (30 hpf: n=65/66; 55 hpf: n=48/53). (E-J) mRNA in situ hybridisation analysis of aldh1a2 expression at 55 hpf in sibling and lamb1aΔ25 mutant embryos, either uninjected (E,H), injected with tp53 MO (F,I) or co-injected with tp53 MO and tnnt2a MO (G,J). The upregulation of aldh1a2 expression in the endocardium of lamb1aΔ25 mutants (H: n=12/14; I: n=12/16) is lost upon injection with tnnt2a MO (J: n=12/18). Black-dashed outlines indicate the heart. Scale bars: 50 μm. A, atrium; V, ventricle. |